Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 14, 2006; 12(42): 6797-6805
Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6797
Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6797
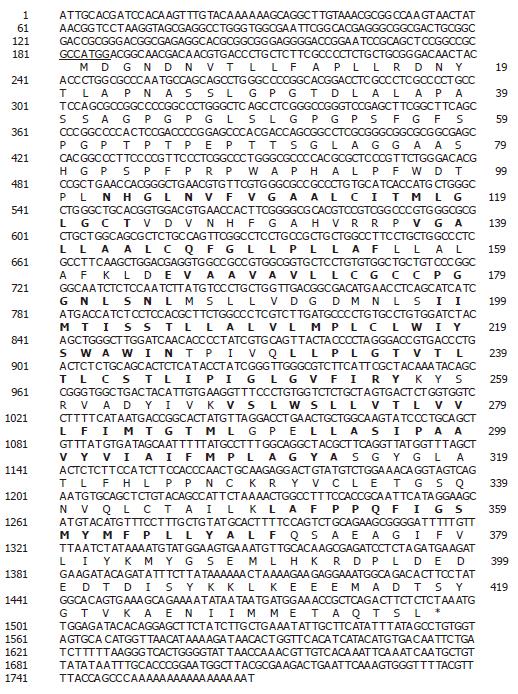
Figure 1 Analysis of the SLC10A4 DNA and protein sequence.
Nucleotide and deduced amino acid sequences of SLC10A4 cDNA. The nucleotide sequence is numbered on the left and the amino acid sequence is numbered on the right. The 5’ UTR encompasses nucleotides (1 to 183) and the initiator methionine lies in an appropriate Kozak sequence (underline). The transmembrane domain is highlighted in bold font. The location of the stop codon is indicated by the asterisk.
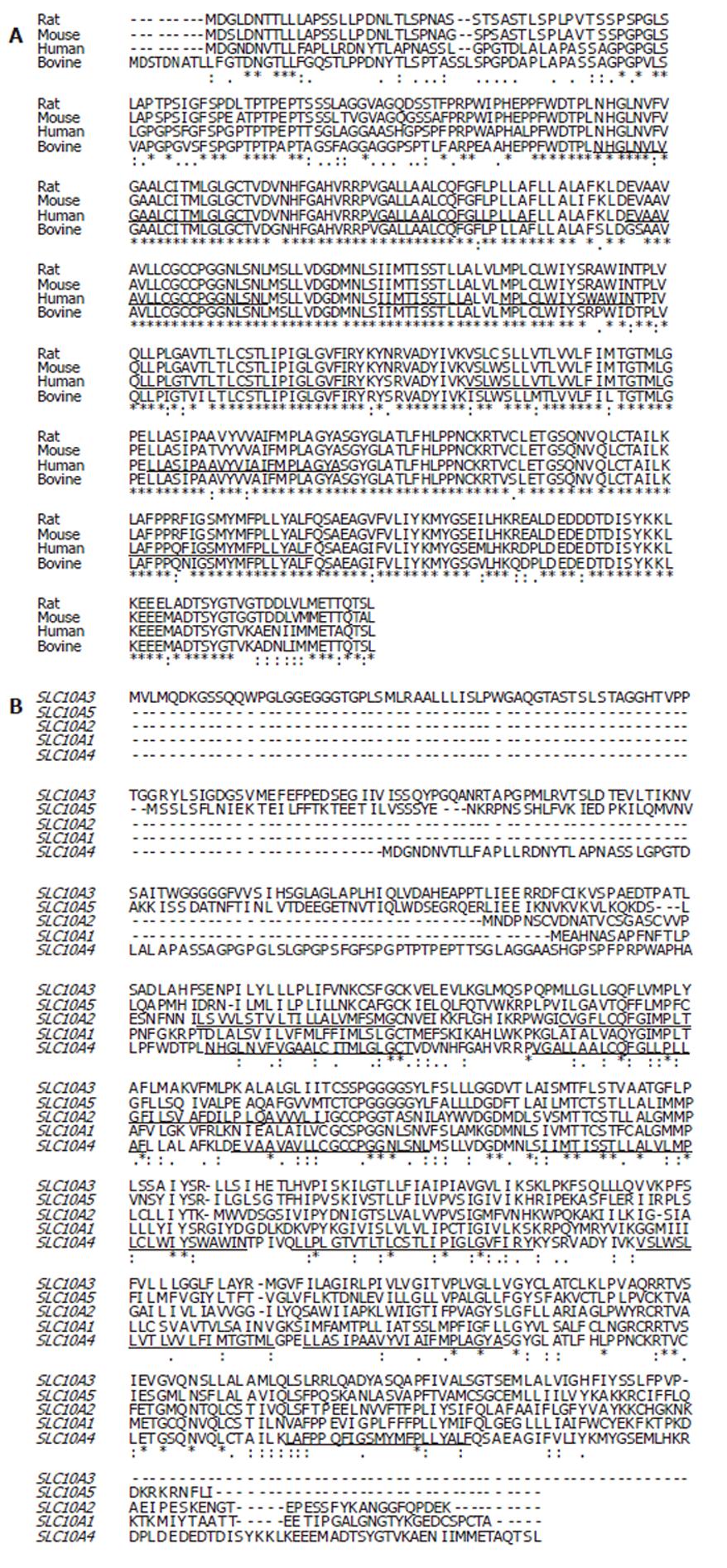
Figure 2 Multiple alignment of the deduced amino acid sequence for SLC10A4 with sequences from rat (XP_579196), mouse (XP_775579), and bovine (XP_591836) and sequences with other SLC10A4 family members (A).
The deduced amino acid sequence of SLC10A4 was aligned with human SLC10A3 (P09131), human SLC10A5 (XP_376781), human SLC10A2 (NP_000443) and SLC10A1 (AAH74724)(B). The SLC10A4 putative transmembrane domains are underlined.
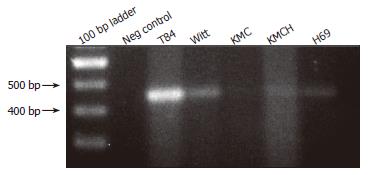
Figure 3 Expression of SLC10A4 mRNA in cultured cholangiocyte.
Positive bands were detected in normal human cholangiocytes (H69) cells, in cholangiocarcinoma cell-lines (Witt, KMC, and KMCH) and the positive control (T84 cells). No bands were detected in the negative control (i.e., no template cDNA).
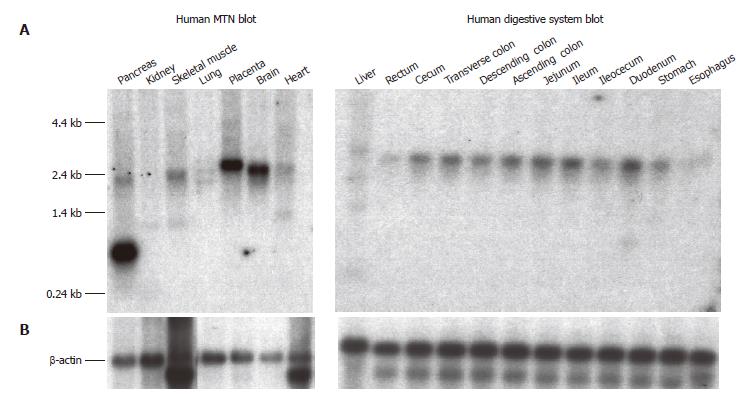
Figure 4 Northern blot analysis of SLC10A4 mRNA expression in human tissues.
(A) The Human MTN and Human Digestive System blots contain 2 μg and 1 μg of poly A+ mRNA per lane, respectively. Each blot was hybridized with a 32P-labeled SLC10A4 specific probe. (B) Hybridization using a 32P-labeled β-actin specific probe to confirm equal loading of mRNA.
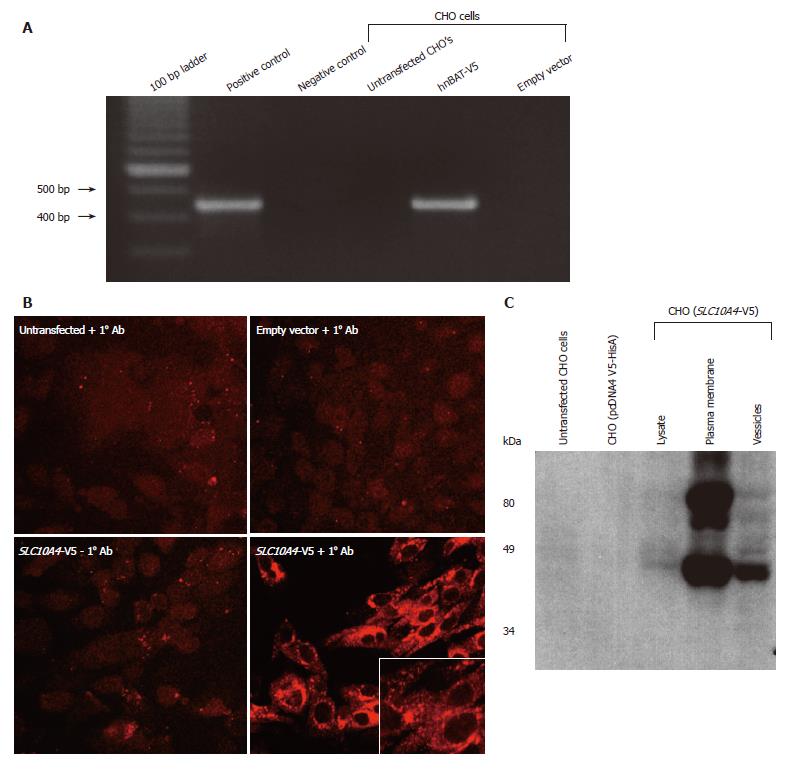
Figure 5 Analysis of the SLC10A4 stably transfected CHO cells.
(A) Expression of SLC10A4 mRNA in transfected CHO cells. SLC10A4 mRNA was detected by RT-PCR in the SLC10A4 transfected cells but not in untransfected CHO cells or the negative control (i.e., no template cDNA). (B) Protein expression of SLC10A4 in stably transfected CHO cells. No staining was found in untransfected CHO cells or CHO cells stably transfected with the empty vector (pcDNA4 V5-HisA) alone in the presence of the affinity-purified monoclonal V5 antibody, or in the CHO cells transfected with SLC10A4 in the absence of the primary antibody. Robust staining was detected in CHO cells transfected with SLC10A4 in the presence of the V5 antibody (× 40). (C) Immunoblotting analysis of SLC10A4 in transfected CHO cells. SLC10A4 is detected in the cell lysate (20 μg), mixed plasma membranes (40 μg) and vesicle (40 μg) fractions of the cells. No bands were seen in cell lysates (40 μg) from untransfected CHO cells or empty vector (pcDNA4 V5-HisA) stably transfected CHO cells.
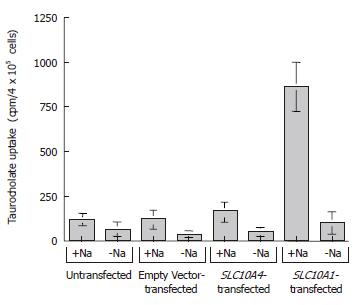
Figure 6 Taurocholate uptake studies by SLC10A4.
The cells were exposed to 200 μmmol/L of taurocholate (a mixture of [3H]taurocholate and unlabeled taurocholate) in the presence or absence of Na+. After 5 min at 37°C, the cells were washed with ice-cold wash solution, lysed, and the amount of [3H] taurocholate was measured. The results are expressed as mean ± SE (n = 3).
- Citation: Splinter PL, Lazaridis KN, Dawson PA, LaRusso NF. Cloning and expression of SLC10A4, a putative organic anion transport protein. World J Gastroenterol 2006; 12(42): 6797-6805
- URL: https://www.wjgnet.com/1007-9327/full/v12/i42/6797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i42.6797