Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 7, 2006; 12(41): 6652-6657
Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6652
Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6652
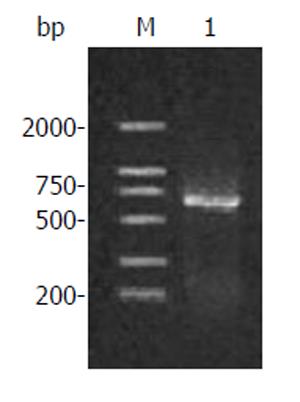
Figure 1 Amplification products of human canstatin.
Lane M: DNA marker; Lane 1: RT-PCR products.
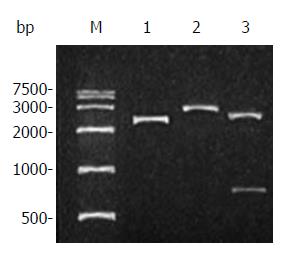
Figure 2 Restriction analysis of plasmid pUCm-T/canstatin.
Lane M: DNA marker; Lane 1: plasmid pUCm-T/ canstatin; Lane 2: pUCm-T/ canstatin digested by BamHI; Lane 3: pUCm-T/canstatin digested by HindIII.
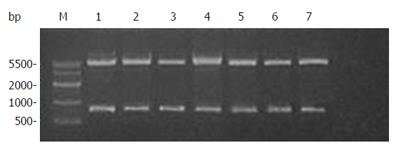
Figure 3 Restriction analysis of plasmid pET-22b(+)/canstatin.
Lane M: DNA marker; Lanes 1 to 7: plasmid DNAs of seven selected colonies digested by both BamHI and HindIII.
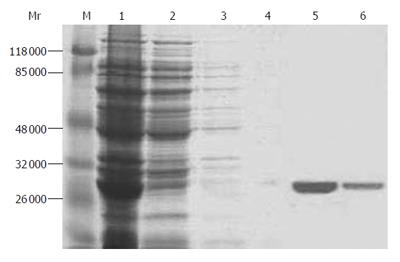
Figure 4 SDS-PAGE gel electrophoresis of purified protein.
Lane M: protein marker; lane 1: total bacterial protein; lane 2: 10 mmol/L imidazole elution; lane 3: 25 mmol/L imidazole elution; lane 4: 50 mmol/L imidazole elution; lane 5: 125 mmol/L imidazole elution; lane 6: 250 mmol/L imidazole elution.
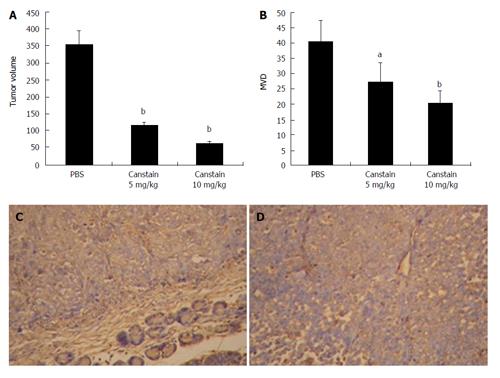
Figure 5 In vivo antitumor effects in a xenograft model.
The in vivo antitumor effect of the canstatin was analyzed in a SW1990 human pancreatic cancer cell orthotopic xenograft model. A: Tumor volumes in different groups: Canstatin 5 mg/kg or 10 mg/kg treatment showed a significantly stronger anti-tumor effect, compared with the PBS treated group (bP < 0.01); B: MVD in xenograft models: significant inhibition of angiogenesis was observed in groups treated with canstatin 5 mg/kg or 10 mg/kg compared with that in the control group (aP < 0.05, bP < 0.01, respectively); C: CD34 immunohistochemistry in group treated with PBS (× 200): tumor sections showed extensive angiogenesis; D: CD34 immunohistochemistry in group treated with canstatin (× 200): tumor sections showed obviously decreased new vessels with a small focus of necrosis.
- Citation: He XP, Li ZS, Zhu RM, Tu ZX, Gao J, Pan X, Gong YF, Jin J, Man XH, Wu HY, Xu AF. Effects of recombinant human canstatin protein in the treatment of pancreatic cancer. World J Gastroenterol 2006; 12(41): 6652-6657
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6652