Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Sep 21, 2006; 12(35): 5635-5643
Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5635
Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5635
Figure 1 Flow cytometric analysis of IGF-1R and EGFR protein expression in HT-29 (A) and HCT-116 (B) cells.
Cells were stained with antibodies against either IGF-1R (black areas) or EGFR (grey areas). Black lines: isotypic controls.
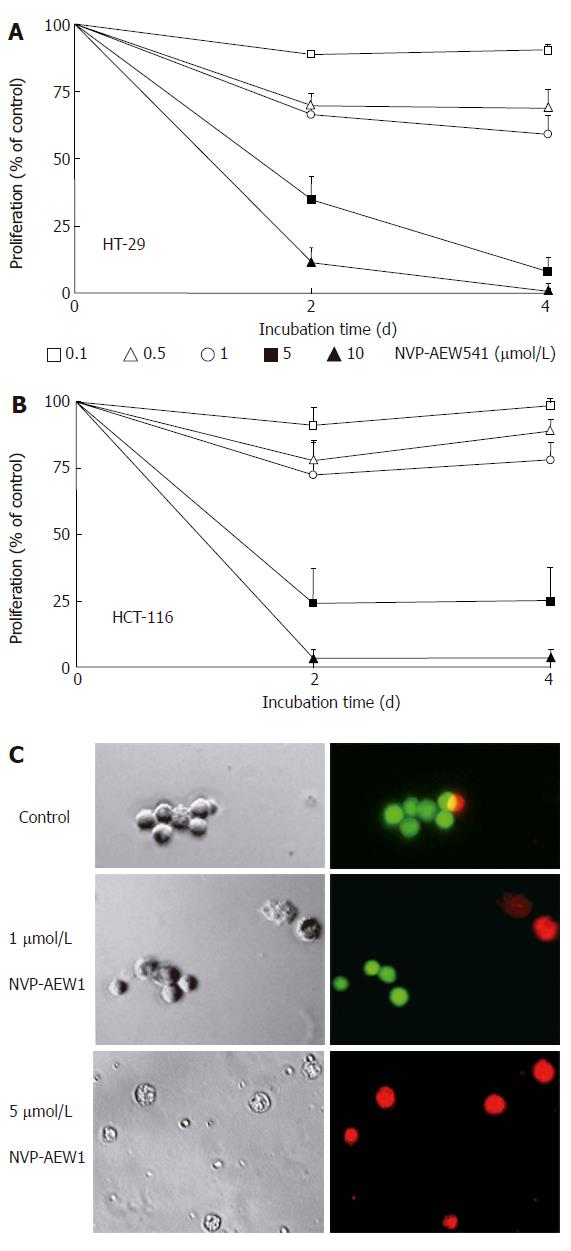
Figure 2 Effects of NVP-AEW541 on HT-29 (A) and HCT-116 (B) cell growth as well as induction of cell death and morphological changes of isolated primary colorectal cancer cells (C).
After 4 d of incubation with rising concentrations of NVP-AEW541, the number of HT-29 (A) and HCT-116 (B) cells decreased by > 95%, as determined by crystal violet staining (mean ± SE, n = 4). In both cell lines statistical significance (P < 0.05) of growth inhibition by NVP-AEW541 was shown for concentrations of 0.5-10 μmol/L. After 3 d of incubation with 0-5 μmol/L NVP-AEW541, the induction of cell death and morphological changes of isolated primary colorectal cancer cells was determined by Live/Dead-fluorescence microscopy (C). Viable cells are stained green, while cells with impaired cell membrane appear red. Phase-contrast images and corresponding fluorescence micrographs of a representative preparation (out of 6 NVP-AEW541-sensitive primary cell cultures) are depicted.
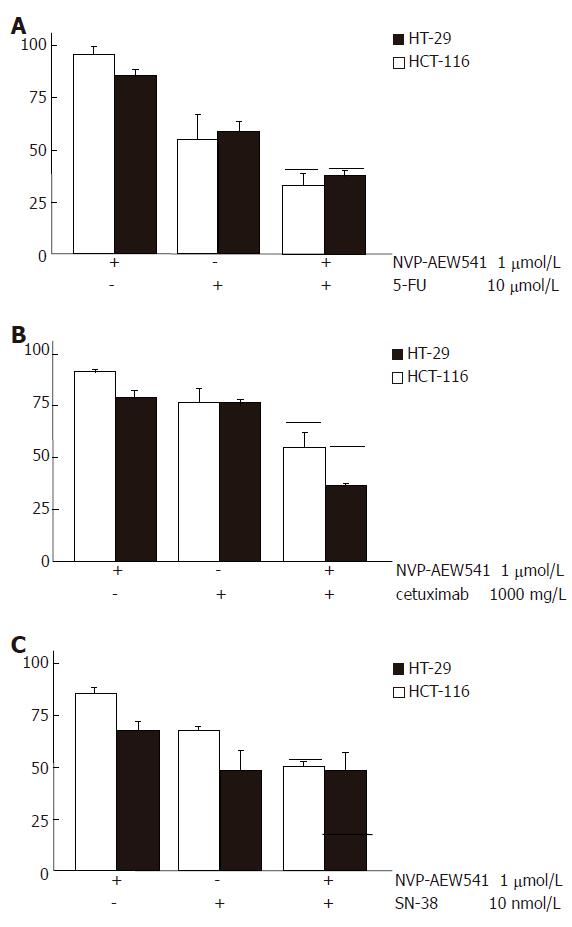
Figure 3 Augmented growth inhibition of combination treatment with NVP-AEW541 plus either 5-FU (A), or SN-38 (B) or cetuximab (C) (mean ± SE, n = 4-6).
A: Combination treatment with sub-IC50 concentrations of NVP-AEW541 plus 5-FU led to synergistic growth inhibition of colorectal cancer cells; B: Combination treatment with sub-IC50 concentrations of NVP-AEW541 plus the humanized EGFR-antibody cetuximab resulted in slightly over-additive antiprolifertive effects; C: Co-treatment with sub-IC50 concentrations of NVP-AEW541 and SN-38 resulted in additive growth inhibition of HCT-116 cells, while no additive growth inhibition was detected in HT-29 cells. Black bars indicate the values of the calculated additive growth inhibition. Data are given as percentage of untreated controls.
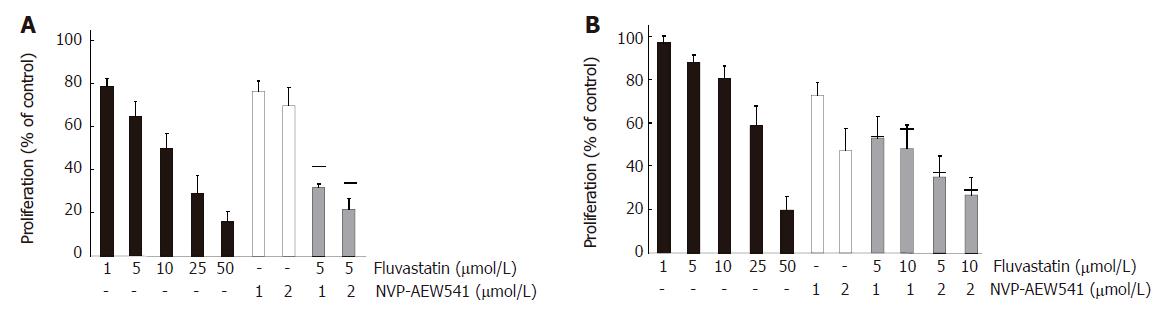
Figure 4 Additive growth inhibition by NVP-AEW541 plus fluvastatin (mean ± SE, n = 3-5).
Fluvastatin (1-50 μmol/L) induced a dose-dependent growth inhibition of HCT-116 (A) and HT-29 (B) cells by > 80% when applied for 3 d. Moreover, combination treatment with sub-IC50 concentrations of fluvastatin and NVP-AEW541 led to (over-)additive growth inhibition after 3 d of treatment. Black bars indicate the values of the calculated additive growth inhibition.
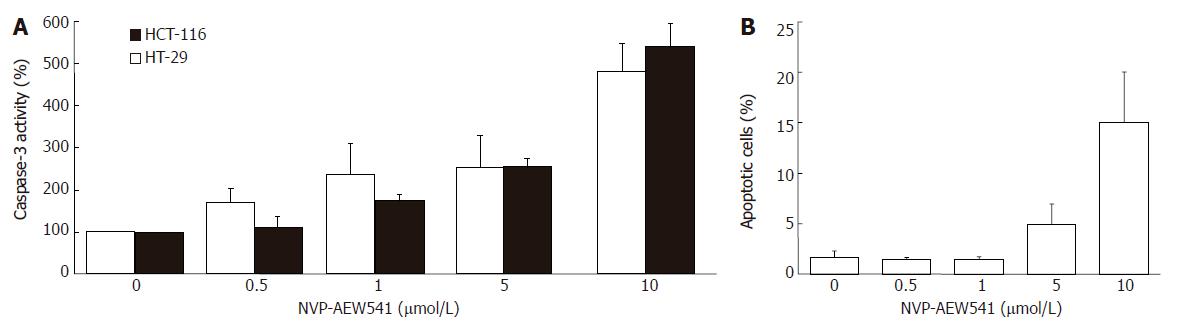
Figure 5 Apoptosis induction by NVP-AEW541 (mean ± SE, n = 4).
A: After HT-29 and HCT-116 cells were incubated with 0.5-10 μmol/L NVP-AEW541 for 24 h, a dose-dependent induction in caspase-3 activation was observed; B: After 72 h of NVP-AEW541 treatment an increased proportion of apoptotic cells measured as subdiploidy was observed in HT-29 cells. aP < 0.05 vs untreated controls.
Figure 6 Effects of NVP-AEW541 on the cell cycle of colorectal carcinoma cells (means ± SE, n = 4).
After 24 h incubation with NVP-AEW541, a dose-dependent accumulation of HT-29 (A) and HCT-116 (B) cells was observed in the G0/G1 phase of the cell cycle (white bars). Proportion of cells in the S and G2/M phase (grey and black bars) decreased. aP < 0.05 vs untreated controls.
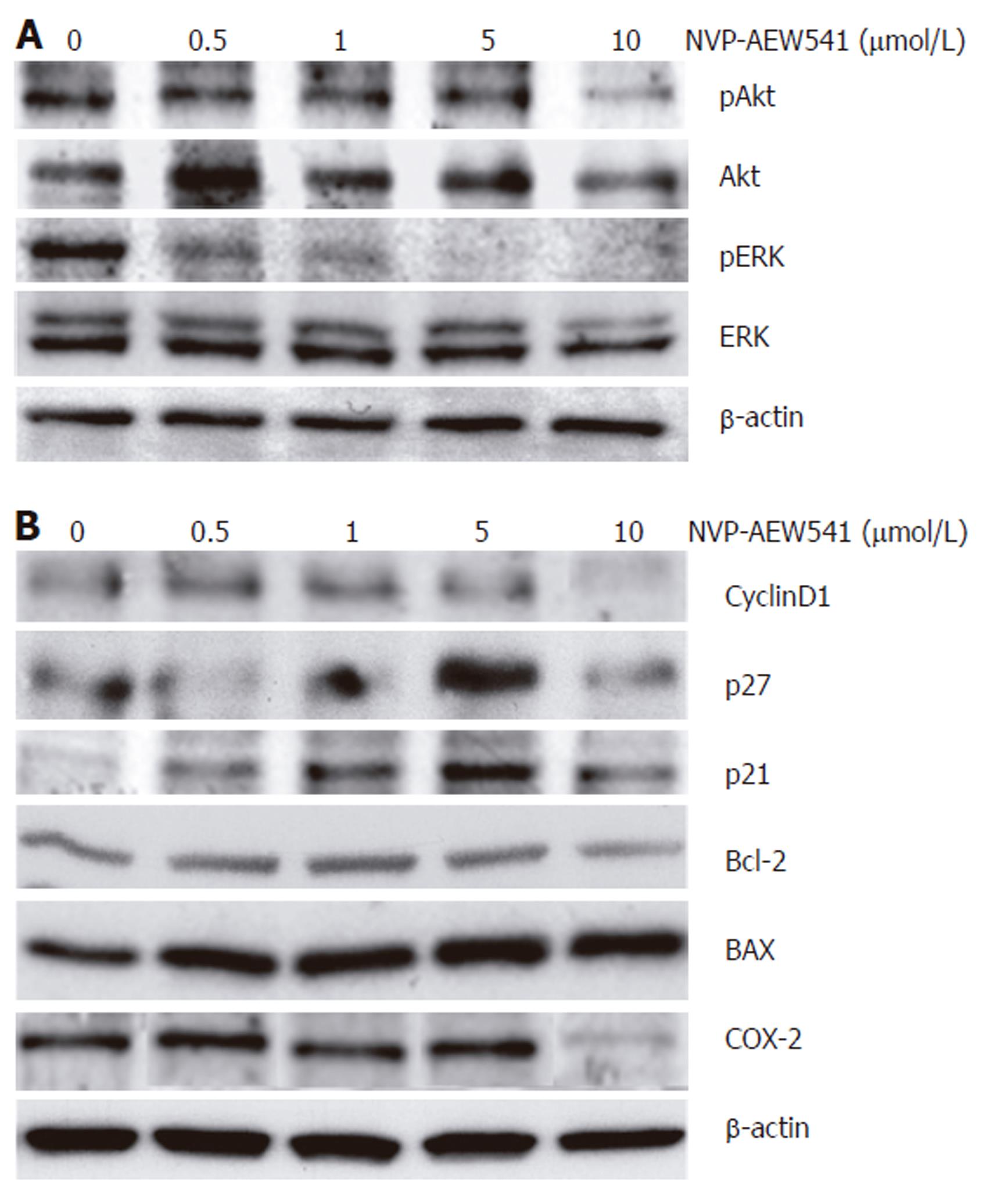
Figure 7 Effects of NVP-AEW541 on the expression and phosphorylation of apoptosis- and cell cycle- related proteins.
Modulation of protein phosphorylation or protein expression by NVP-AEW541 treatment was analyzed by Western blotting. A: NVP-AEW541 treatment (24 h) induced a dose-dependent dephosphorylation of mitogenic ERK1/2; B: NVP-AEW541 dose-dependently increased the expression of the proapoptotic Bax protein, while the expression of the antiapoptotic protein Bcl-2 slightly decreased. The cell cycle promoter cyclin D1 was down-regulated by NVP-AEW541 treatment, while the cell cycle inhibitors p21Waf1/CIP1 and p27Kip1 were up-regulated. Moreover, COX-2 expression was down-regulated by NVP-AEW541. Expression of β-actin was used as a loading control.
- Citation: Höpfner M, Sutter AP, Huether A, Baradari V, Scherübl H. Tyrosine kinase of insulin-like growth factor receptor as target for novel treatment and prevention strategies of colorectal cancer. World J Gastroenterol 2006; 12(35): 5635-5643
- URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5635.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5635