Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Jun 14, 2006; 12(22): 3512-3522
Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3512
Published online Jun 14, 2006. doi: 10.3748/wjg.v12.i22.3512
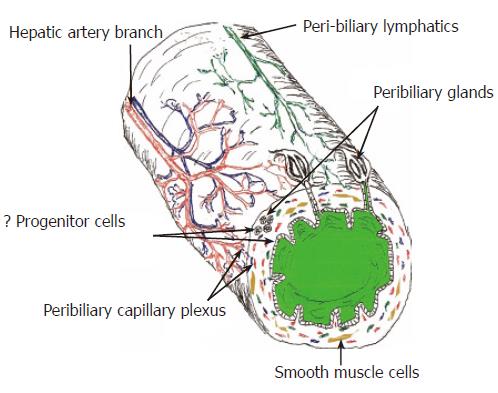
Figure 1 Diagram of the extra-hepatic and large intra-hepatic bile ducts highlighting some important anatomic and physiologic considerations that can potentially impact wound healing.
Except for the peribiliary glands and some features of the BEC, small intra-hepatic bile ducts have a similar anatomy. All of the bile ducts, including the small intra-hepatic bile ducts are supplied only by the hepatic artery and the peribiliary vascular plexus, shown in red. All bile ducts also contain either smooth muscle cells and/or facultative myofibroblasts in their wall. Deep wounds to the biliary tree result in activation and/or transformation of myofibroblasts that greatly increase the risk of wound contraction, fibrosis, and stricture formation.
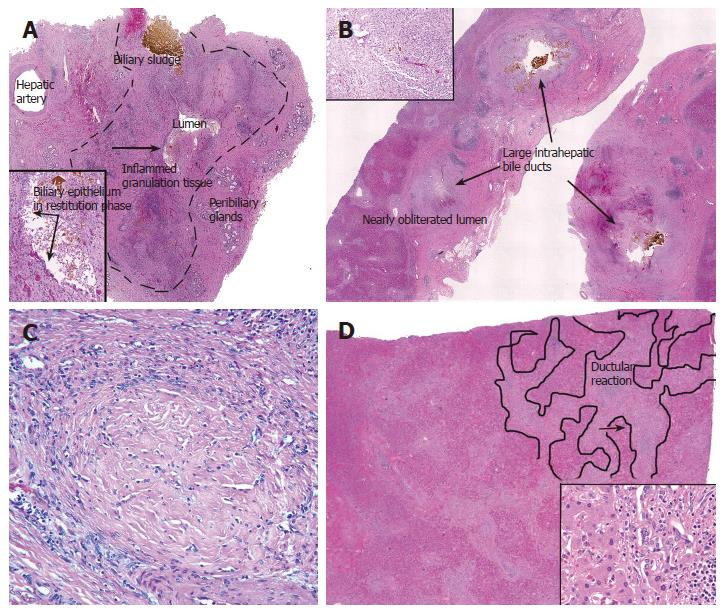
Figure 2 A: Cross-section of an extra-hepatic bile duct (outlined by dashed lines) from a liver allograft that failed because of the biliary sludge syndrome.
Note the hepatic artery branch traveling along the outer wall, the peribiliary glands, mucosal ulceration, and that the lumen is nearly obliterated. Ulceration exposes the underlying stroma to bile, which results in inflammation, granulation tissue, and myofibroblast activation and proliferation in the underlying stroma. The inset shows at higher magnification the area near the arrow; note the bile sludge, BEC in restitution phase, and stromal inflammation and granulation tissue (arrows); B: Cross-section of several large intra-hepatic bile ducts from the same liver. Note that the same processes are occurring in these ducts. The duct lumen in the lower left is nearly obliterated by inflamed granulation tissue and myofibroblasts (inset); C: The final stages of “pathologic wound healing” in the intra-hepatic ducts can result in complete fibrous obliteration of the bile duct lumen by concentric rings of fibrous tissue, as shown here; D: Sections from the periphery of the same liver show a prominent ductular reaction consisting of BEC and periductal myofibroblasts (inset). This occurs because of increased pressure in the biliary tree distal to the site of luminal obliteration. Notice that the cholangioles and myofibroblasts form a wedge of tissue that arises from the portal tract and distorts the liver architecture (outlined on the right side of image).
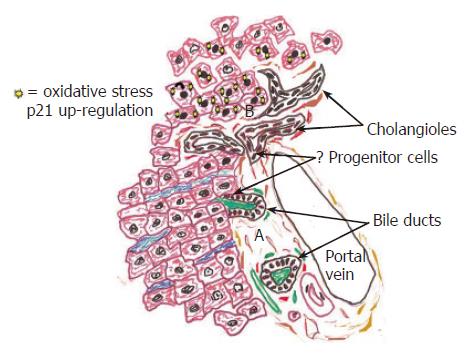
Figure 3 This diagram illustrates the peripheral aspects of the biliary tree, including the portal tract and periportal hepatic parenchyma in normal (A: bottom of figure) and in diseased livers (B: top of figure).
A: Rare liver progenitor, or stem cells, are thought to reside within the Canals of Herring, or the portion of the bile ductules that connects BECs to hepatocytes. The same close relationship between BEC, the arterial supply, and periductal myofibroblasts seen in the large ducts continues to the biliary tree periphery, as shown here. B: During chronic necro-inflammatory liver diseases a variety of insults, such as cholestasis[1], HCV replication[114,115], steatosis[2,116] copper deposition[117], and alcohol[118] can cause oxidative stress, preferentially in hepatocytes. The stress upregulates hepatocyte nuclear p21 expression (illustrated by solid nuclei at top of diagram), which in turn, inhibits hepatocytes proliferation and accentuates ductular reactions[1,2]. A close relationship between BEC and periductal myofibroblasts in the smallest bile ductules usually results in co-activation of these populations recognized as ductular reactions, which often precede the development of cirrhosis.
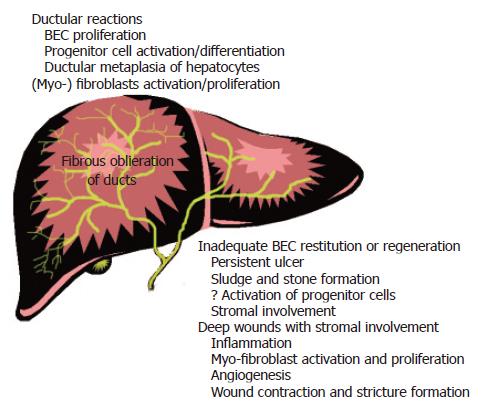
Figure 4 Summary of the most common “wound repair” reactions that contribute to the development of chronic liver disease and liver cancers.
Although all of the processes described in the text can occur at any site in the biliary tree, certain types of responses are more common at certain sites. For example, in the extra-hepatic and large intra-hepatic bile ducts (lower right), inadequate or ineffectual epithelial restitution or proliferation results in persistent ulcers and stone and sludge formation. This, in turn, leads to possible activation of progenitor cells and stromal involvement, which can also be triggered by “deep wounding” of the large ducts. Stromal involvement is usually accompanied by inflammation, angiogenesis, and activation and proliferation of myofibroblasts. All of these processes contribute to wound contraction and stricture formation, which is one of the most common and clinically significant problems in the large ducts.
- Citation: Demetris AJ, III JGL, Specht S, Nozaki I. Biliary wound healing, ductular reactions, and IL-6/gp130 signaling in the development of liver disease. World J Gastroenterol 2006; 12(22): 3512-3522
- URL: https://www.wjgnet.com/1007-9327/full/v12/i22/3512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i22.3512