Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Jan 28, 2005; 11(4): 469-475
Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.469
Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.469
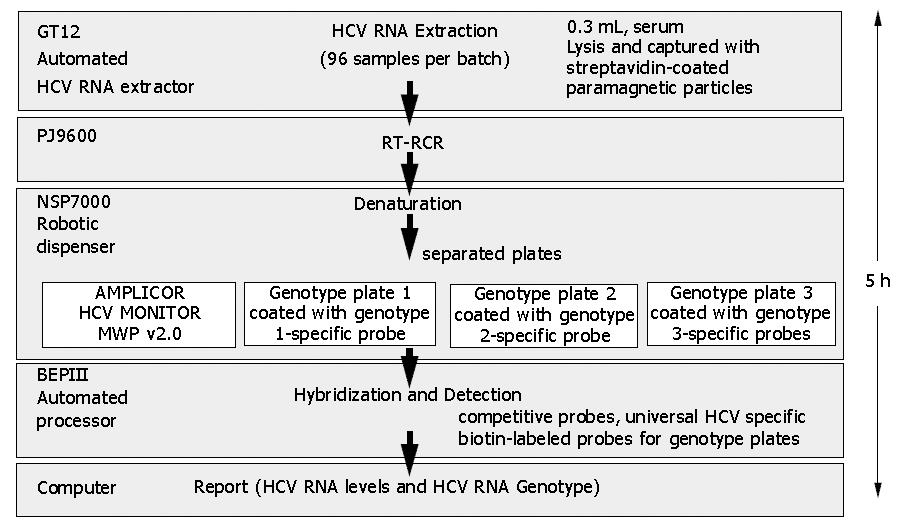
Figure 1 HCV Guideline test flow.
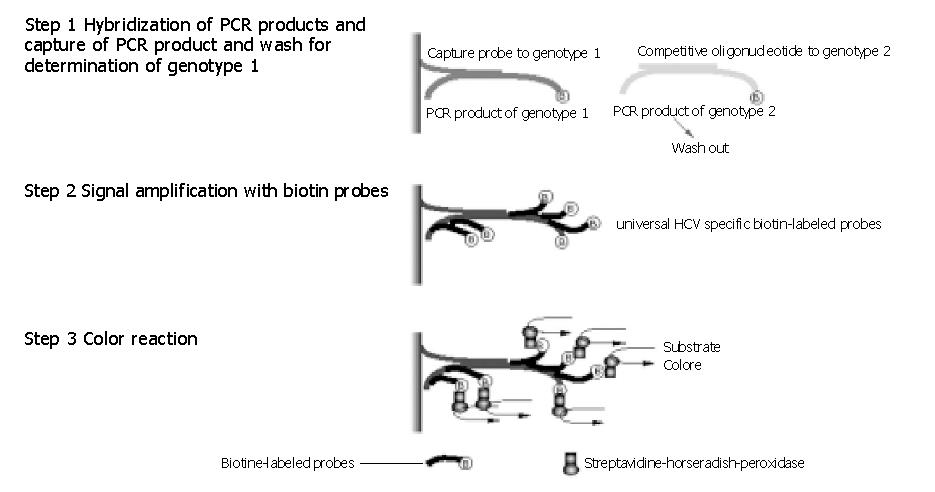
Figure 2 Strategy of HCV Guideline test.
Step 1: The PCR product (here genotype 1) was hybridized to the specific capture probe, immobilized on a micro-plate. Other PCR products (e.g. of genotype 2) did not hybridize to the immobilized probe and were captured by the competitive oligonucleotide probes. The genotype specific-hybridized PCR product is retained on the micro-plate, while other PCR products were washed out from the plate. Step 2: Additional universal biotin-labeled-probes hybridized to the specific PCR product resulting in signal amplification. Step 3: Biotin reacted with streptavidine which was conjugated to the horseradish-peroxidase (HRP) enzyme. The color reaction was measured after the addition of enzyme specific substrates.
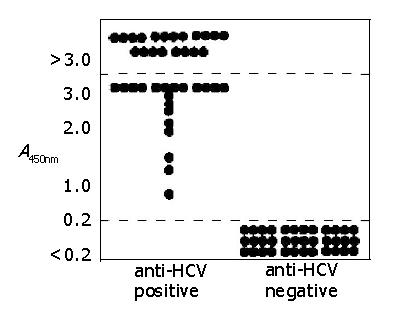
Figure 3 Specificity of HCV Guideline test.
36 anti-HCV-positive samples and 36 anti-HCV-negative samples were used to determine the specificity of the HCV Guideline test. All the anti-HCV-positive samples showed an absorbance greater than 0.2, whereas all anti-HCV-negative samples showed an absorbance below 0.2.
- Citation: Mukaide M, Tanaka Y, Kakuda H, Fujiwara K, Kurbanov F, Orito E, Yoshioka K, Fujise K, Harada S, Kozaki T, Takemura K, Hikiji K, Mizokami M. New combination test for hepatitis C virus genotype and viral load determination using Amplicor GT HCV MONITOR test v2.0. World J Gastroenterol 2005; 11(4): 469-475
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.469