Copyright
©The Author(s) 2005.
World J Gastroenterol. Sep 7, 2005; 11(33): 5156-5161
Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5156
Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5156
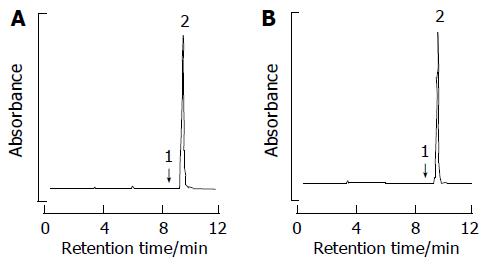
Figure 1 Reverse-phase HPLC separation of amygdalin by phosphate buffer.
(A) D-amygdalin standard. (B) D-amygdalin obtained by our method; peaks: 1, neoamygdalin; 2, D-amygdalin.
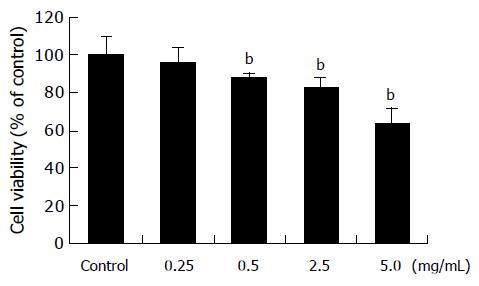
Figure 2 Cytotoxicity of amygdalin.
SNU-C4 human colon cancer cells were treated with various concentrations (0.25, 0.5, 2.5, and 5 mg/mL) of amygdalin for 24 h prior to the determination of cellular viability through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Independent experiment was repeated thrice. Results are presented as mean±SE (bP<0.01 vs control group).
Figure 3 Expression pattern in a 8k human cDNA microarray.
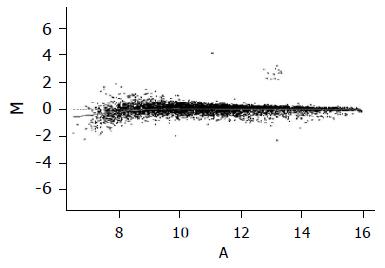
Figure 4 Scattered plot of the normalization results by global M method.
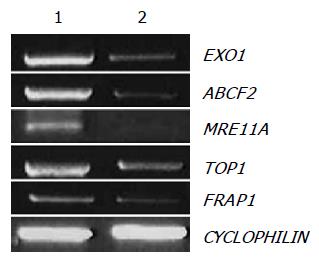
Figure 5 Confirmation of cDNA microarray results of downregulated genes by RT-PCR.
Five genes, exonuclease 1 (EXO1), ABC, sub-family F (GCN20), member 2 (ABCF2), MRE11 meiotic recombination 11 homolog A (MRE11A), topoisomerase (DNA) I (TOP1), and FK506 binding protein 12-rapamycin associated protein 1 (FRAP1), were analyzed by RT-PCR with total RNA from control and amygdalin (5 mg/mL, 24 h)-treated human colon cancer cells. As an internal control, CYCLOPHILN was amplified.
- Citation: Park HJ, Yoon SH, Han LS, Zheng LT, Jung KH, Uhm YK, Lee JH, Jeong JS, Joo WS, Yim SV, Chung JH, Hong SP. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J Gastroenterol 2005; 11(33): 5156-5161
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5156.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5156