Copyright
©The Author(s) 2005.
World J Gastroenterol. Aug 7, 2005; 11(29): 4547-4551
Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4547
Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4547
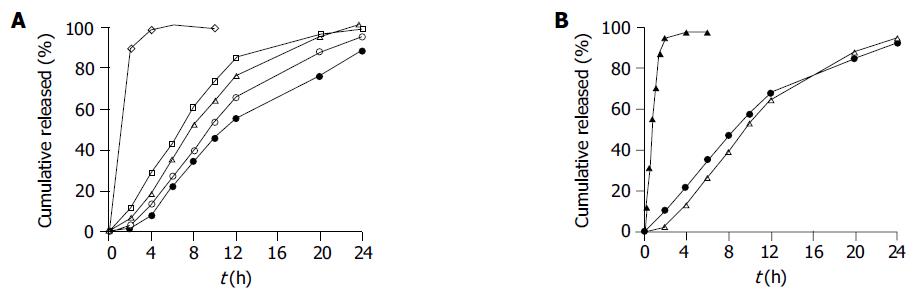
Figure 1 Effect of different coating levels on SM·HCl release behavior from the coated pellets in distilled water (n = 6).
A: Pellets coated at five levels of 0% (◇), 3% (□), 5% (△), 6.5% (○), 8% (●, w/w, total solid applied); B: rapid-release pellets (▲) and slow-release pellets at a 6.5% coating level (△) and a mixture of slow- and rapid-release pellets (9:1, w/w, ●).
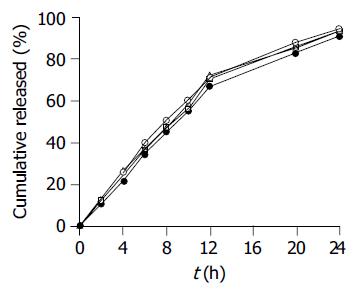
Figure 2 Effect of pH of the dissolution medium on SM•HCl release from coated pellets of the optimal formulation (n = 6).
Distilled water (○), 0.1 mol/L HCl (●), pH 6.8 (△) and pH 7.4 (□) PBS.
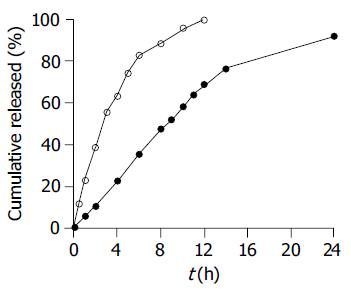
Figure 3 Comparative SM•HCl release profiles of 24-h test sustained-release pellets (●) and 12-h reference sustained-release tablets (○, n = 6).
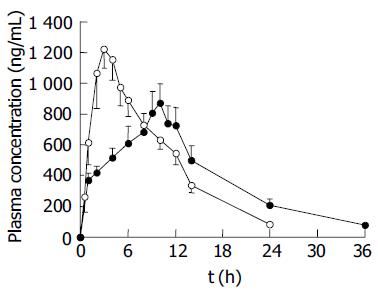
Figure 4 SM•HCl plasma concentration-time profiles after oral administration of 24-h test sustained-release pellets (●) and 12-h reference sustained-release tablets (○) in beagle dogs at a single dose of 120 mg/animal.
Each value represents mean±SE (n = 6).
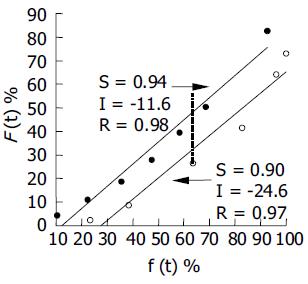
Figure 5 Relationship between cumulative SM•HCl percentage release in vitro f(t) and percentage absorption in vivo F(t) of the two dosage forms.
S, I and R are the abbreviations for the slope, intercept and correlation coefficient for the correlation equation, respectively. Twenty-four-hour test sustained-release pellets (●) and 12-h reference sustained-release tablets (○).
- Citation: Sun J, Shi JM, Zhang TH, Gao K, Mao JJ, Li B, Sun YH, He ZG. Impact of release characteristics of sinomenine hydrochloride dosage forms on its pharmacokinetics in beagle dogs. World J Gastroenterol 2005; 11(29): 4547-4551
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4547