Copyright
©The Author(s) 2005.
World J Gastroenterol. Jul 7, 2005; 11(25): 3887-3892
Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3887
Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3887
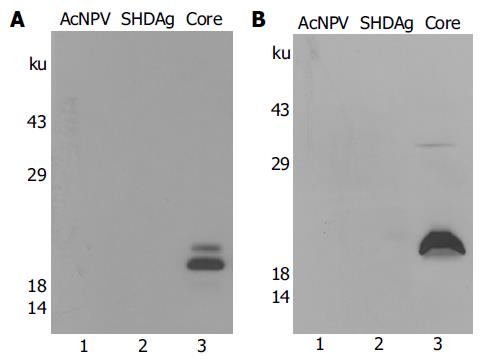
Figure 1 Core proteins are secreted into the culture medium in insect cells.
A: Expression of HCV core protein in insect cells. cDNA corresponding to the HCV core was subcloned into the BamHI site of the transfer vector pVL941 behind polyhedrin promoter. Recombinant baculoviruses expressing HCV core protein were produced as described in Materials and methods. Sf9 cells were infected with either wild type (AcNPV) or recombinant baculoviruses expressing SHDAg, or recombinant baculoviruses expressing HCV core protein and were harvested at d 3 postinfection. Cell lysates were separated by SDS-containing polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane. Proteins were detected by Western blotting using HCV patient sera. Lane 1, Sf9 cells infected with wild type baculoviruses; lane 2, Sf9 cells infected with recombinant baculovirus expressing SHDAg; lane 3, Sf9 cells infected with recombinant baculovirus expressing HCV core; B: The culture medium from (A) was centrifuged at 3 500 r/min for 15 min to remove cell debris. Supernatant was further centrifuged at 12 000 g for 30 min to remove baculoviruses. The resultant supernatant was then pelleted through a 300 g/L sucrose cushion for 90 min at 27 000 g. The pellet was dissolved in sample buffer and analyzed by Western blotting using HCV patient sera.
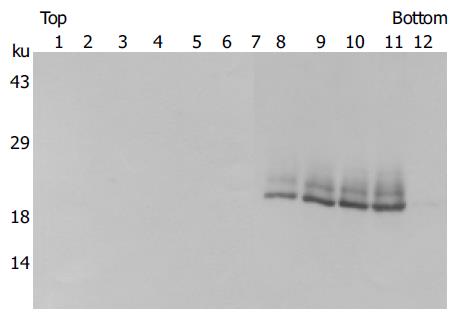
Figure 2 Isolation of core proteins from cell culture supernatant.
Sf9 cells were infected with recombinant baculoviruses expressing HCV core protein. The culture supernatant was collected at d 3 postinfection. Following removal of cell debris and recombinant baculoviruses, the released core proteins were partially purified through a sucrose cushion and were subjected to velocity gradient centrifugation. Twelve fractions were collected and proteins were detected by Western blot analysis using HCV patient sera.
Figure 3 Kinetics of core protein secretion in culture medium of the recombinant baculovirus-infected insect cells.
Sf9 insect cells were infected with recombinant baculoviruses expressing full-length core protein (A). Cell lysates were prepared from d 1 to d 4 postinfection, and separated by SDS-PAGE on a 15% gel and Western blotted with an HCV patient serum (B); Culture supernatants were harvested from d 1 to d 4 postinfection and secreted core proteins were detected by Western blot analysis. Kinetics of intracellular core protein (C) and extracellular core protein (D) productions were quantified using a densitometric scanner (Molecular Dynamics).
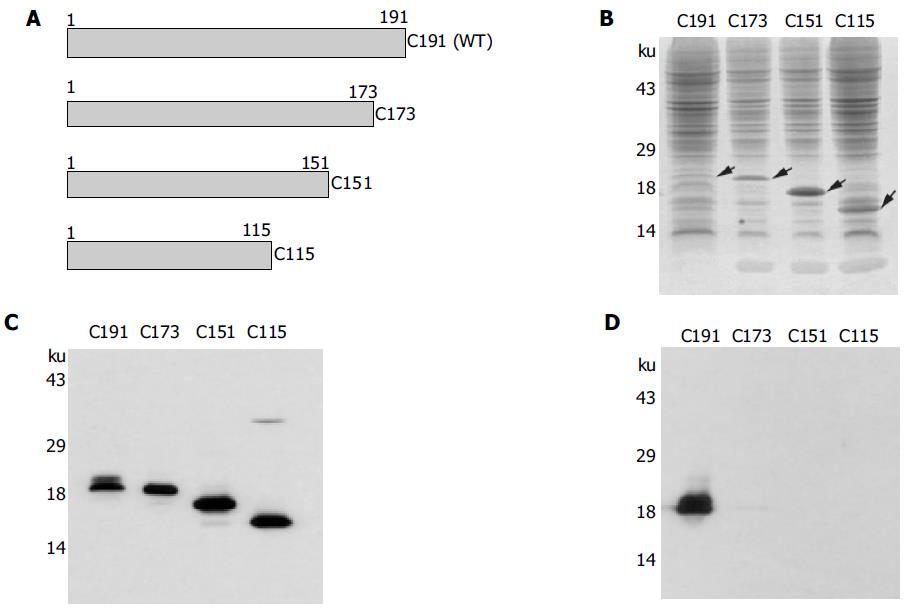
Figure 4 Effects of mutant core proteins on core secretion.
(A) Schematic diagram illustrating the recombinant baculoviruses expressing truncated forms of core protein. Mutant core constructs were generated by PCR and the subsequent recombinant baculoviruses were made as described in Materials and methods. Protein expression of each mutant was confirmed by SDS-PAGE and Coomassie Brilliant Blue staining (B) and Western blot analysis by using HCV patient serum (C). (D) Determination of extracellular core release among wild type and mutant core proteins. Insect cells were infected with recombinant baculoviruses expressing wild type and various mutant forms of core proteins and harvested at d 3 postinfection. Culture supernatants were partially purified and determined for core secretion by Western blot analysis as described in the legend to Figure 1B.
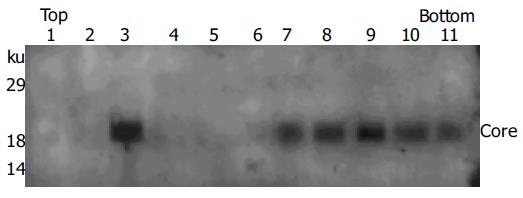
Figure 5 Membrane flotation analysis of secreted core proteins.
Sf9 insect cells were infected with recombinant baculoviruses expressing full-length HCV core protein. Culture supernatant was collected at 60 h postinfection and secreted core proteins were partially purified as described in Materials and methods. The sample was then subjected to fractionation by equilibrium sucrose gradient centrifugation. Eleven fractions collected from the top were analyzed by Western blotting using rabbit anti-HCV core antibody.
- Citation: Choi SH, Park KJ, Kim SY, Choi DH, Park JM, Hwang SB. C-terminal domain of hepatitis C virus core protein is essential for secretion. World J Gastroenterol 2005; 11(25): 3887-3892
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3887