Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Jun 28, 2005; 11(24): 3772-3777
Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3772
Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3772
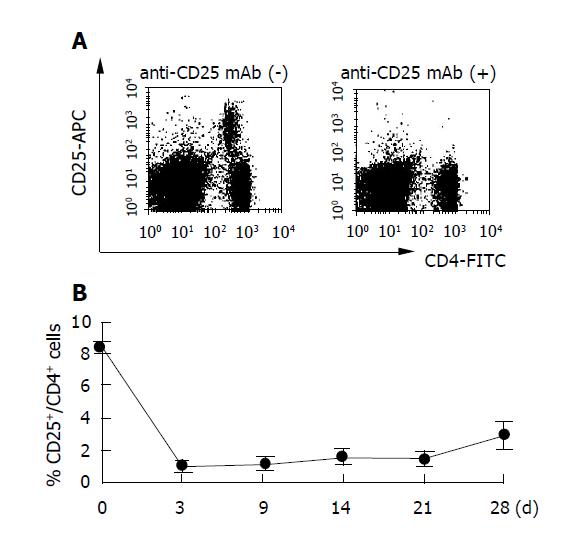
Figure 1 Injection of anti-CD25 mAb results in selective loss of CD25+ cells.
Mice (six mice per group) were injected with 500 μg of anti-CD25 mAb (PC61) and analyzed by flow cytometry at the indicated days after treatment. Representative responses of PBMCs from one mouse are shown (A). Data are expressed as a percentage of CD25+CD4+ cells in the CD4+ cell population in peripheral blood. Three experiments were performed with similar results (B). Data are shown as the mean±SD of six mice tested individually n = 6.
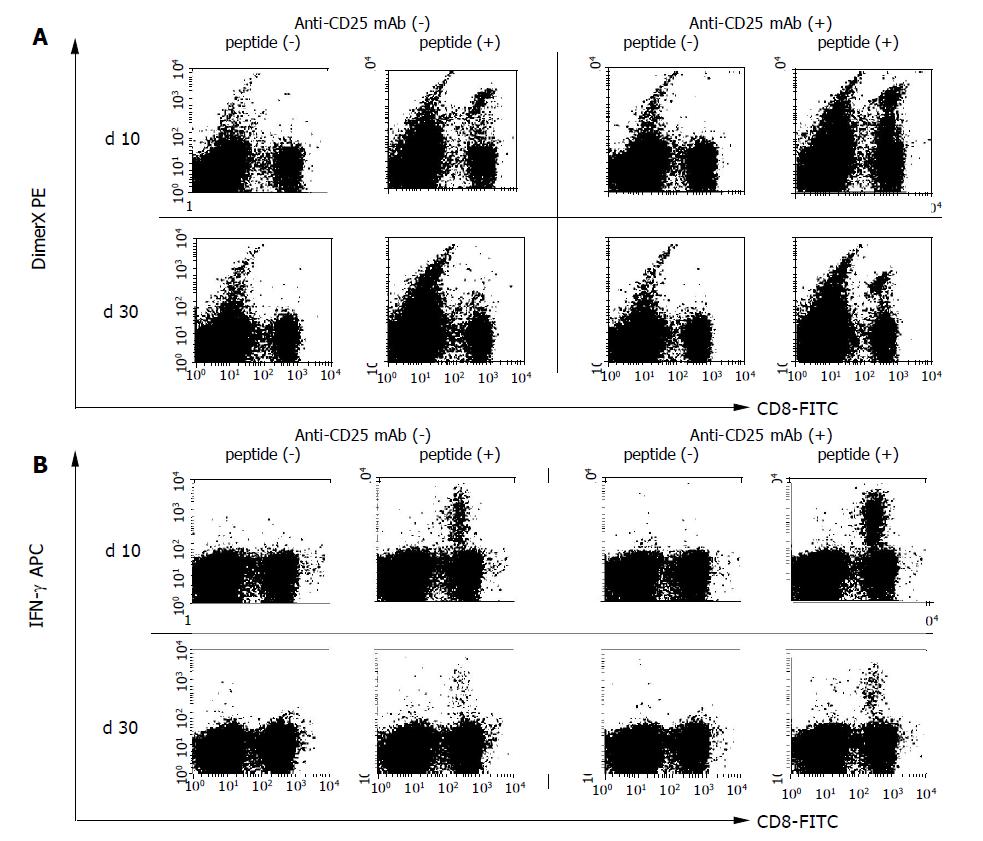
Figure 2 Flow cytometry analysis to detect HBV-specific CD8+ T cells.
Mice (six mice per group) were immunized by HBV plasmid DNA with or without anti-CD25 mAb treatment to eliminate CD25+ cells. PBMCs were harvested and the presence of HBV-specific CD8+ T cells were examined by flow cytometry on the 10 and 30 d after DNA immunization. A: HBV-specific CD8+ T cells were detected by S28-39 peptide loaded DimerX staining. Peptide unloaded empty DimerX was used as a control. B: Intracellular IFN-γ staining was performed to detect functional HBV S23-39 specific CD8+ T cells. PBMCs were incubated with 1 μg/mL of S28-39 peptide for 6 h and stained for the production of IFN-γ. Three experiments were performed with similar results. Representative responses of PBMCs from one mouse are shown in each panel.
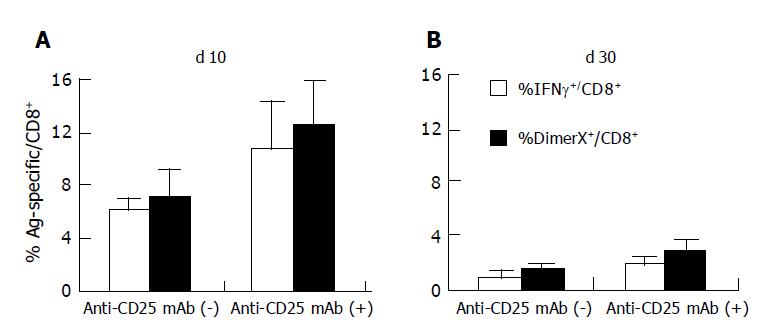
Figure 3 Frequency of HBV-specific CD8+ T cells induced by DNA immunization.
The frequency of S28-39-specific CD8+ T cells was detected by DimerX staining and intracellular IFN-γ staining as described in Figure 2. The data (mean±SD of PBMCs from six mice) show the percentages of DimerX positive or IFN-γ producing cells in the CD8+ cell population (A and B). The data presented are representative of three individual experiments n = 6.
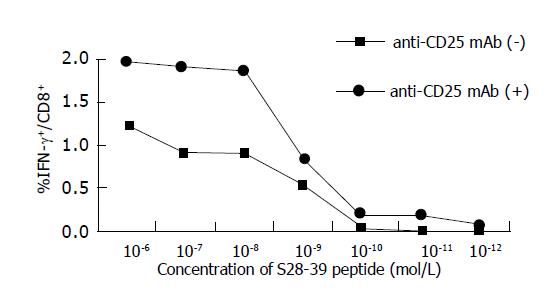
Figure 4 Functional avidity of S28-39-specific CD8+ T cells.
The functional avidities of the HBV S28-39-specific CD8+ T cells were compared in a peptide dose titration experiment and the ability to secrete IFN-γ upon stimulation with S28-39 peptides was determined. PBMCs from six mice were pooled and incubated for 6 h with indicated concentrations of S28-39 peptide and stained for CD8 and IFN-γ. The data show percentages of IFN-γ producing cells in the CD8+ cell population.
- Citation: Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J Gastroenterol 2005; 11(24): 3772-3777
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3772.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3772