Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Apr 14, 2005; 11(14): 2080-2087
Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2080
Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2080
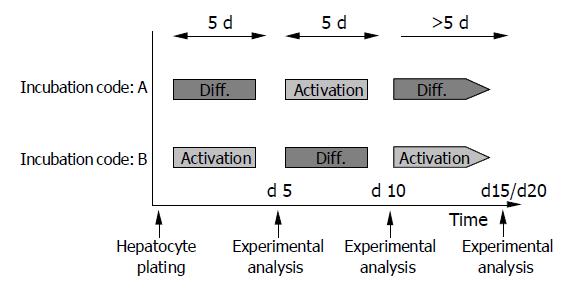
Figure 1 Experimental design.
HC were alternately cultured in AM or DM. Environmental switch was carried out on d 5 and 10 after plating. Incubation code A confers to initial incubation in DM, code B to initial incubation in AM. Experimental analysis of HC was carried out on d 5, 10, 15, and 20, if not otherwise indicated.
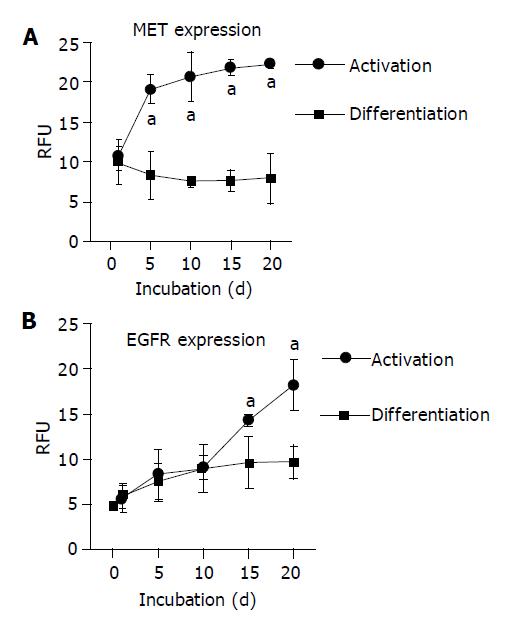
Figure 2 Expression kinetics of growth factor receptors.
A: MET expression; B: EGFR expression. HC were permanently cultured either in a AM or in a DM. The receptor level was detected by flow cytometry and expressed as relative fluorescence units (RFU). mean±SD (n = 6), aP<0.05 between groups.
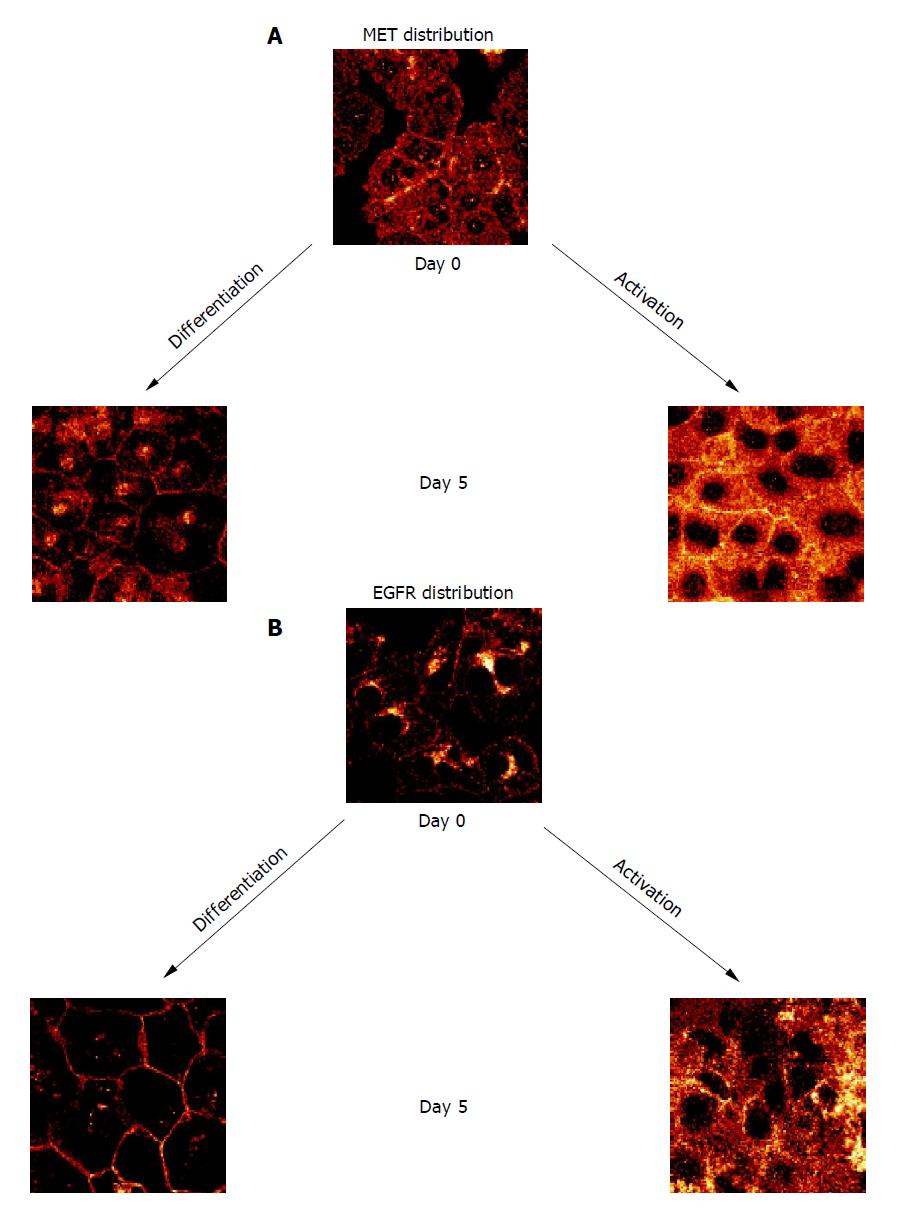
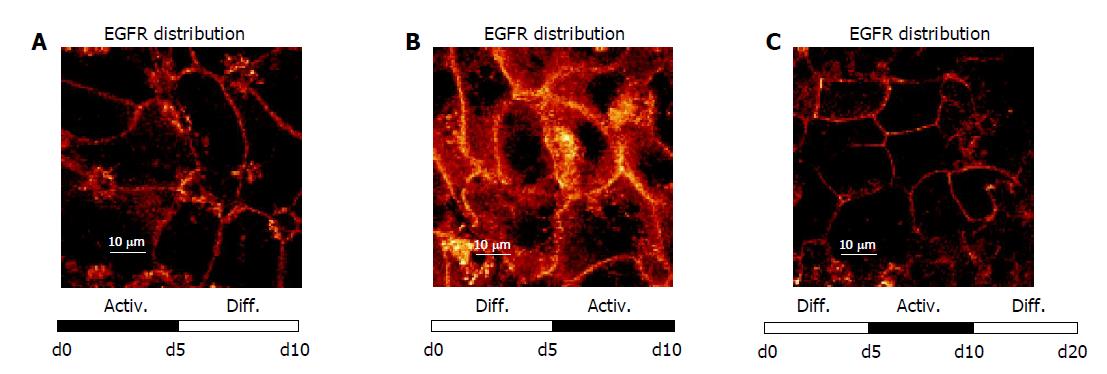
Figure 3 Confocal analysis of distribution pattern of growth factor receptors on d 0 and 5 after culture onset.
A: MET distribution; B: EGFR distribution. HC were cultured either in DM or in AM. Indocarbocyanine staining, ×100/1.3 oil immersion objective. Each figure is representative for three separate experiments.
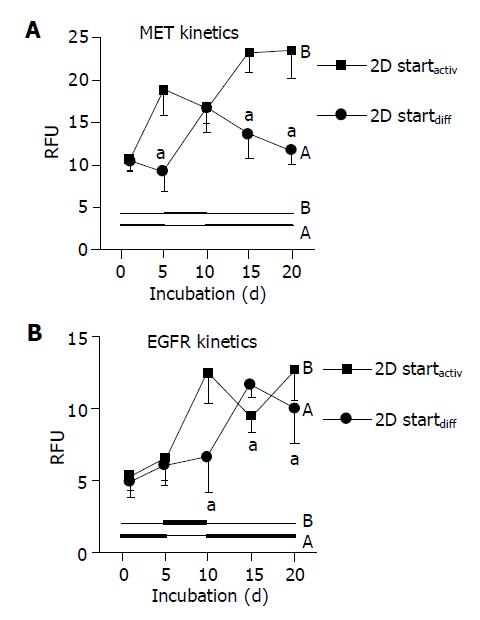
Figure 4 Dynamics of growth factor receptor expression.
A: MET kinetics; B: EGFR kinetics. HC were plated on a 2D collagen matrix and incubated alternately in AM or DM. Code A (2D startdiff) is related to initial 5-d incubation in DM, followed by 5-d incubation in AM and subsequent incubation in DM. Code B (2D startactiv) concerns the incubation order AM-DM-AM. The receptor level was detected by flow cytometry and expressed as RFU. mean±SD (n = 6), aP<0.05 between groups.
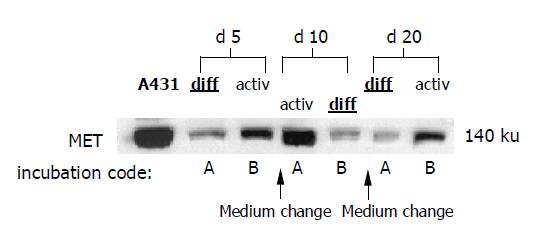
Figure 5 Western blot analysis of MET protein content.
HC were incubated alternately in AM or DM. Medium was changed on days 5 and 10. Incubation code A indicates initial 5-d incubation in DM, followed by 5-d incubation in AM and subsequent incubation in DM. Incubation code B is related to initial incubation in AM, followed by incubation in DM and, subsequently, in AM. A431 cells served as positive controls. The blots are representative for three separate experiments.
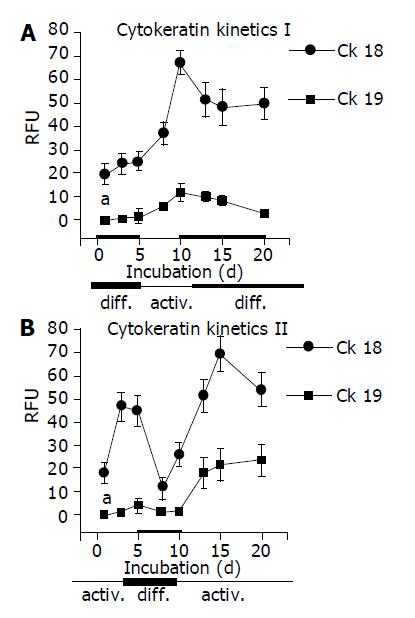
Figure 6 CK 18 and 19 expression, several time points after cell plating.
A: Kinetic part I is related to initial 5-d incubation in DM, followed by 5-d incubation in AM and subsequent incubation in DM; B: Kinetic part II is related to the incubation order AM-DM-AM. HC were seeded on a 2D collagen matrix and incubated alternately in AM or DM. CK level was detected by flow cytometry and expressed as RFU. mean±SD (n = 6); aP<0.05 between groups.
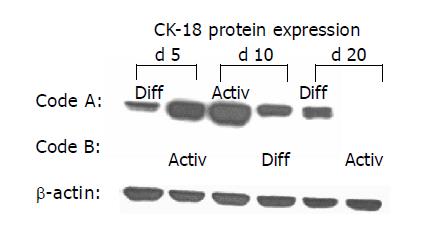
Figure 7 Western blot analysis of CK 18 protein content.
HC were incubated alternately in AM or DM. Medium was changed on d 5 and 10. Code A indicates initial 5-d incubation in DM, followed by 5-d incubation in AM and subsequent incubation in DM. Code B is related to initial incubation in AM, followed by incubation in DM and, subsequently, in AM. β-actin served as the internal control. The blot is representative for three separate experiments.
Figure 8 Dynamics of EGFR distribution depending on modulation of the culture environment.
A: Receptor localization along the plasma membrane after switching the extracellular milieu from de-differentiation (activation) to differentiation inducing conditions (d 10); B: The reverse order (DM-AM) evoked a distinct receptor translocation into the cytoplasm (d 10); C: Receptor re-localization onto the cell surface was induced again after a further 10-d incubation in DM (d 20). Confocal microscopy, ×100/1.3 oil immersion objective. Each figure is representative for three separate experiments.
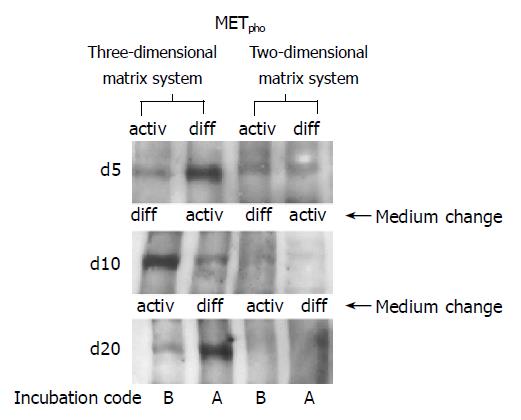
Figure 9 Western blot analysis of milieu dependent shift of MET phosphorylation (METpho).
Environmental switch has been carried out on d 5 and 10. Left lanes indicate HC cultivation within a 3D collagen matrix (sandwich), right lanes confer to a 2D culture system (collagen matrix). Incubation code A indicates initial 5-d incubation in DM, followed by 5-d incubation in AM and subsequent incubation in DM. Incubation code B is related to initial incubation in AM, followed by incubation in DM and subsequent incubation in AM. The blots are representative of three separate experiments.
- Citation: Auth MK, Boost KA, Leckel K, Beecken WD, Engl T, Jonas D, Oppermann E, Hilgard P, Markus BH, Bechstein WO, Blaheta RA. Controlled and reversible induction of differentiation and activation of adult human hepatocytes by a biphasic culture technique. World J Gastroenterol 2005; 11(14): 2080-2087
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2080