Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Mar 21, 2005; 11(11): 1658-1662
Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1658
Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1658
Figure 1 Predicted amino acid sequences of the defective or wild type HDAg expressed by the plasmids.
Dashes indicate conserved amino acids. The terminations of the wild type and defective HDAgs are indicated by asterisks (1). The coiled-coil domain is doubly underlined. The nuclear localization signal is marked by a thin line. The RNA-binding domain is marked by a thick line. The large HDAg package signal is marked by a hatched bar. The small and large forms of wild type HDAg are referred to as HDAg-S and HDAg-L, respectively. Similarly, HDAg-L-18d, HDAg-S-53d and HDAg-S-13d represent the defective HDAgs derived from the same patient. HDAg-L-18d had an insertion of a nucleotide G in the coding region of HDAg and resulted in frameshift and premature stop translation of HDAg due to the generation of a novel stop codon. HDAg-S-53d contained a segment (nt 1255 to 1329) of deletion and substituted by a segment (nt 337 to 355) from different regions of the HDAg-coding sequence. This mutation also resulted in frameshift and premature stop translation of HDAg. HDAg-S-13d had an insertion of two cytosines between the first and the second stop codons of HDAgs. This mutation resulted in translation of a wild type small HDAg, and a frameshift translation of large HDAg.
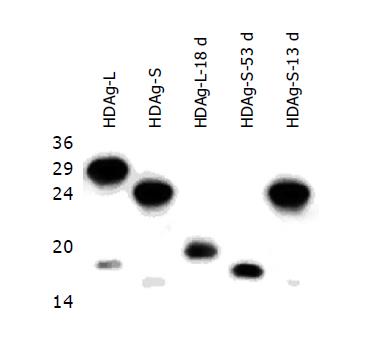
Figure 2 Western blot analysis of wild type and defective HDAgs.
The wild type and defective expression plasmids of HDAg (see Methods and Figure 1) were transfected into the Huh-7 hepatoma cell line. Cellular proteins were analyzed by Western blotting. Wild type small and large HDAgs (HDAg-S and HDAg-L) were in proper positions and served as controls. Defective HDAgs (HDAg-L-18d and HDAg-S-53d) were of different molecular weights due to mutations. HDAg-S-13d had a mutation between the stop codons for the small and the large HDAg. Therefore, the small HDAg was expressed and was electrophoretically correct.
- Citation: Wu JC, Hsu SC, Wang SY, Huang YH, Sheen IJ, Shih HH, Syu WJ. “Defective” mutations of hepatitis D viruses in chronic hepatitis D patients. World J Gastroenterol 2005; 11(11): 1658-1662
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1658