Published online Dec 26, 2017. doi: 10.13105/wjma.v5.i6.132
Peer-review started: September 16, 2017
First decision: November 7, 2017
Revised: November 21, 2017
Accepted: December 3, 2017
Article in press: December 3, 2017
Published online: December 26, 2017
Processing time: 101 Days and 6.1 Hours
To determine the incidence and risk factors for mechanical complications (MC) after surgical correction of adult spinal deformity (ASD) with osteotomy.
A retrospective study was performed. Inclusion criteria: Surgical correction of ASD using osteotomy; male or female; > 20 years old; follow-up ≥ 24 mo or revision. The MC of spine and spinal instrumentation were studied separately. Risk analysis included assessment of the association between more than 50 different characteristics (demographic, clinical, radiographic, and instrumentation) with different types of MC.
The medical records of 94 operations in 88 subjects were analyzed: Female (68%), mean age 58.6 (SD, 12.7) years. Cumulative incidence of MC at 2 year follow-up was 43.6%. Of these, 78% required revision (P < 0.001). The following characteristics had significant (P ≤ 0.05) association with MC: (1) Preoperative: osteoporosis, smoking, previous spinal operation, sagittal vertical axis (SVA) > 100 mm, lumbar lordosis (LL) < 34°; (2) postoperative: SVA > 75 mm; operative correction: SVA > 75 mm, LL > 30°, thoracic kyphosis > 25°, and pelvic tilt > 9°; a fall; pseudarthrosis; and (3) device and surgical technique: use of previously implanted instrumentation; use of domino and/or parallel connectors; type of osteotomy (PSO vs SPO) if preoperative SVA < 100 mm; lumbar osteotomy location; in-situ rod contouring > 60°; and fixation to sacrum/pelvis.
Risk of MC after surgical correction of ASD is substantial. To decrease this risk over- and/or insufficient correction of the sagittal imbalance should be avoided.
Core tip: The main study goal was evaluation of incidence and risk factors for different mechanical complications (MC) after surgical correction of adult spine deformity with osteotomy. Around half of patients experienced complications during two postoperative years; 78% of these cases required additional surgery. MC of spine occurred earlier and more often required revision than the MC of spinal instrumentation. The main risk factors for MC included severe preoperative sagittal imbalance, inadequate correction of the spinopelvic alignment, preoperative comorbidities (osteoporosis), postoperative events (falls), and features of the spinal instrumentation.
- Citation: Barton C, Noshchenko A, Patel VV, Cain CMJ, Kleck C, Burger EL. Different types of mechanical complications after surgical correction of adult spine deformity with osteotomy. World J Meta-Anal 2017; 5(6): 132-149
- URL: https://www.wjgnet.com/2308-3840/full/v5/i6/132.htm
- DOI: https://dx.doi.org/10.13105/wjma.v5.i6.132
Surgical correction of adult spinal deformity (ASD) often requires one or more osteotomies such as a Smith-Petersen (SPO) and/or pedicle subtraction (PSO); SPO involves resection of the posterior column of the spine while PSO utilizes a resection of a triangular wedge through the pedicle and vertebral body[1,2]. In general a PSO can achieve significantly greater correction than a single SPO, and has been utilized for spinal deformities with severe sagittal imbalance, with relatively good clinical outcomes[3-10]. However, this method increases the risk of postoperative complications, which may result in revision surgeries[8-14]. The reported cumulative rate of revisions after surgical correction of ASD ranged from 28% at 24 mo follow-up[15] to 48% at 49 mo follow-up[11], with an increased cost in treatment. It was noted that the majority of these reoperations were related to mechanical complications (MC)[11,16]. Currently, there is no generally accepted definition of MC in spinal surgery. These have been described as failure of the fusion, spine or instrumentation. Therefore, the reported incidence of MC after surgical treatment in ASD is heterogeneous, and varies from 3.7% to 37%[10-12,17-24]. Published data concerning risk factors for MC in ASD corrected with an osteotomy are fragmented and suffer from several limitations. In particular, it was found that rate of postoperative symptomatic pseudarthrosis identified at 2-5 years after PSO was 10.5%. Patients with pseudarthrosis had significantly higher rates of previous fusion surgeries with pseudarthrosis, previous lumbar decompression, preoperative radiation of the spine/sacrum, and a preoperative history of inflammatory arthropathies/neurological disorders[25]. However, the level of corresponding risk was not evaluated. It was also noted that insufficient correction of spinal sagittal alignment with a PSO may be linked to pseudarthrosis and proximal junctional failure (PJF)[26]. However, the level of correction was defined by an integral index, making interpretation of the results difficult, and risk analysis was not performed. The reported incidence of symptomatic rod fracture (RF) after surgical correction of ASD with any osteotomy is cosistent: 5.3%[27], and 6.8%[14]; after a PSO it was higher: 15.8%[14] and 16.2%[27]. The following risk factors of RF were revealed (P ≤ 0.05): Fusion construct crossing the thoracolumbar and lumbosacral junctions (OR = 9.1); maximum sagittal rod contour ≥ 60° (OR = 10.0); application of dominos and/or parallel connectors in the instrumentation constract (OR = 10.0); pseudarthrosis diagnosed at ≥ 1 year follow-up (OR = 28.9); and fixation to pelvis[27]. However, only a limited number of RF cases were included, which could cause an underestimation of the risks associated with other factors.
While the literature lacks clear evidence about the risk factors for MC after osteotomies, we expanded our literature search to MC after surgery for ASD (non-specific to osteotomy). This yielded several factors significantly (P ≤ 0.05) associated with MC. In particular: The number of instrumented segments; fusion to the sacrum; and high preoperative pelvic tilt (PT), > 26°[11]. This was limited as MC were not specified, and were combined with neurological complications. In another study, MC after spinal fusion in ASD were classified as: (1) Proximal junctional complications including fracture of upper instrumented vertebra (UIV) and/or one level above (UIV + 1); and postoperative pseudarthrosis[16]. In this study the following risk factors were revealed: ≥ 3 comorbidities, hazard ratio (HR) = 3.2; smoking, HR = 3.3; and preoperative sagittal imbalance with sagittal vertical axis (SVA) > 95 mm, HR = 2.7[16]. When compiling the data from multiple studies, the following preoperative spinopelvic measurements were identified as risk factors for PJF: SVA > 50 mm, OR = 2.5[28]; thoracic kyphosis (TK) > 30°, HR = 3.2[29-31]; and pelvic incidence (PI) > 55°[29,31]. Risk factors for PJF were also associated with postoperative overcorrection of the spinopelvic alignment, including: Postoperative SVA change[32], in particular, postoperative SVA < 50 mm[33]; change of TK > 10°[34], or > 30°, OR = 2.5[28]; and change of lumbar lordosis (LL) > 30°, OR = 4.8[28], HR = 2.4[31]. Risk factors for PJF associated with instrumentation and surgical technique included: Posterior spinal fusion[32], and fixation to the sacrum or pelvis, OR = 2.2[28,32,33]. Finally, risk of PJF was linked to the following demographic data: Male[8]; age > 55[35-37]; osteoporosis[32]; and increased body mass index (BMI)[35,36]. However, in some of the studies referenced above, the level of risk was not assessed properly. It is unclear how the revealed risk factors can be modified by the implementation of an osteotomy; whether there is a synergistic effect of different risk factors? If normal postoperative SVA < 50 mm is associated with risk of MC[28], what postoperative SVA or combination of factors is associated with less risk? The predictive value of the revealed risk factors was not defined.
The purpose of this study was to evaluate the most clinically relevant MC seen after surgical correction of ASD with corrective osteotomies, taking into account the incidence, period of occurrence, association with additional surgeries, and assessment of risk factors associated with the MC and their predictive value.
After institutional review board approval (COMIRB #14-1258), medical records and radiographic images were retrospectively identified in patients with ASD undergoing surgical correction with an instrumented spinal fusion including one or more osteotomies of the thoracic and/or lumbar spine. These operations were performed between 2007 and 2014 by 4 surgeons at a single institution. Inclusion criteria were used: (1) Demographics: Age > 20 years; gender, male and female; (2) diagnosis of ASD from any of the following etiologies: fixed sagittal imbalance, idiopathic or degenerative scoliosis, kyphosis or kyphoscoliosis, posttraumatic kyphosis, idiopathic or postoperative flat back syndrome, and ankylosing spondyloarthropathies; and (3) operation consisting of ≥ 2 spinal posterior instrumented fusion levels (with or without interbody fusion) of the lumbar, or thoracic spine and having an osteotomy (PSO and/or SPO). All patients had to have follow-up not less than 2 years or undergone revision/reoperation prior to 2 year follow-up. Exclusion criteria consisted of: Malignancy, infection, congenital diseases, acute trauma, or latest follow-up < 2 year (unless revision surgery was performed at any postoperative time-point). If a patient had multiple SPOs during one operation, it was analyzed as one SPO procedure. If an operation included both SPOs and PSO, it was analyzed as one PSO procedure. The final decisions regarding inclusion/exclusion for each case was made by the project principal investigator (EB).
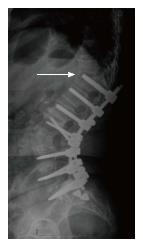
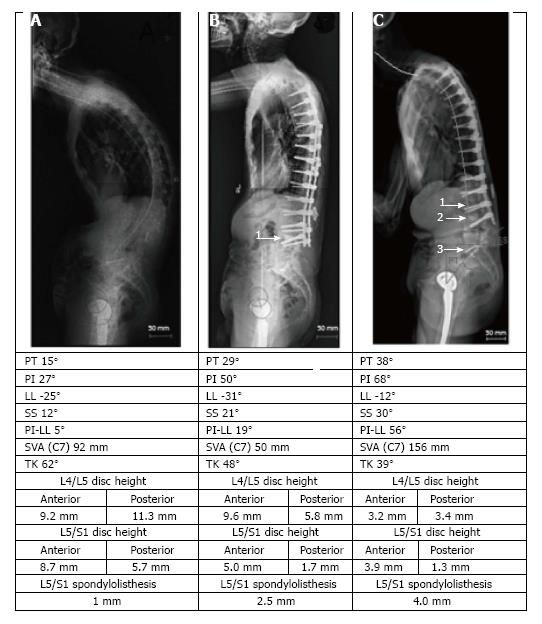
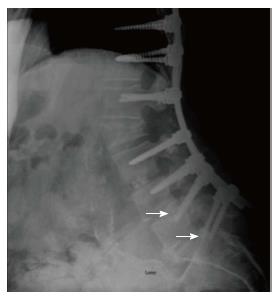
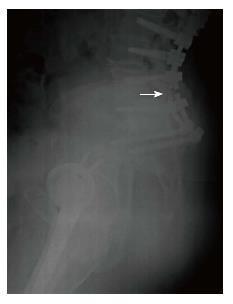
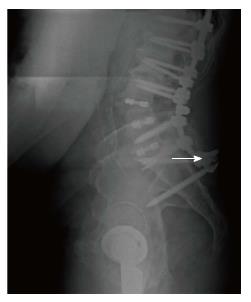
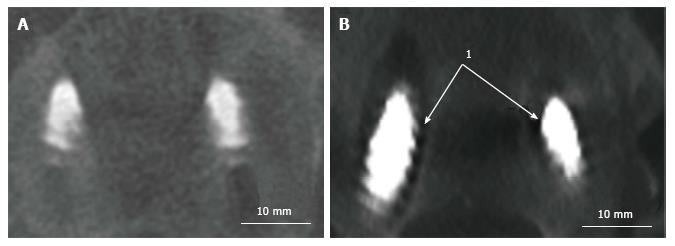
The following MC were taken into consideration: (1) MC of the spine: Vertebral fracture (VF): Single or multiple fractures of the vertebral body, vertebral endplate, and/or pedicle at any level(s) of the spine; PJF: Fracture and/or severe spondylolisthesis of the UIV and/or adjacent vertebra (UIV+1) (Figure 1); Distal segment degeneration/failure (DSF): Vertebral fracture and/or significant spondylolisthesis, collapse of the intervertebral disc(s) with or without herniation, stenosis with neurologic claudication at the lowest instrumented fused level (LIV) or caudally (Figure 2); and (2) MC of instrumentation: Screw loosening (SL): Failure of the bone-screw interface, including screw pull-out from the pedicle, sacrum, or ilium (Figure 3); RF: Fracture of one or both rods (Figure 4); iliac bolt connector failure (IBCF): loosening or fracture; disassociation of instrumentation (DI): Disconnection/loosening between any element(s) of the posterior fusion construct (Figure 5).
All types of MC were diagnosed radiographically and/or during revision surgery. The specific postoperative period when each MC was diagnosed was collected. The following characteristics were collected and assessed as potential risk factors: (1) Demographic: Age, gender, ethnicity, BMI, and smoking status at the time of the index operation; (2) Clinical: Primary diagnosis, indication for the index operation, osteoporosis, history of pseudarthrosis after previous operation (in cases of revision surgery); (3) Characteristics of the studied (index) operation: primary or revision, type of osteotomy (SPO/PSO), osteotomy location, number of posterior levels fused, transition segments of the spine crossed by posterior instrumentation, connection of new instrumentation to indwelling hardware, cement use, use of anterior supplemental fixation, fixation to the sacrum, fixation to the pelvis; (4) Characteristics of the instrumentation: Type of screws (polyaxial/monoaxial), screw manufacturer, type of rods (precontoured by manufacturer/no), rod material, thickness and shape of rods, greatest angle of rod contouring, manufacturer of rods, method of the rod contouring, number of crosslinks, number of domino and/or parallel connectors, use of bone morphogenetic protein (BMP); (5) Radiographic characteristics of spinopelvic alignment in standard preoperative and postoperative (1st-4th week after operation) images: SVA, LL, TK, PT, pelvic incidence (PI), PI-LL mismatch with discrete and absolute differences between postoperative and preoperative values of each spinopelvic parameter; and (6) Postoperative events: Fall after operation, but before diagnosis of MC, and postoperative pseudarthrosis (nonunion at the fused site(s) confirmed radiographically (plane radiography or computed tomography) or during revision at more than 1 year follow-ups) Osteoporosis/osteopenia information was obtained from the patients’ medical records by analyzing the results of dual energy X-ray absorptiometry or ultrasound evaluation.
Clinical and demographic data were extracted from the medical records by an experienced researcher (CB) under control of the project PI (EB). Spinopelvic parameters were defined by retrospective analysis of radiographic images using Surgimap surgical planning software (Nemaris Inc, New York, NY, United States) by a trained researcher (CB). Ten percent of the performed measurements were additionally evaluated by the PI (EB) to assess correspondence. Correspondence between these two measurements was assessed by the Kappa test[38]. Measurements were regarded identical, if deviation between them did not exceed 10% of the smaller value. The studied spinopelvic parameters were defined using currently accepted standards of measurement as outlined in previous studies[39,40]. If the quality of a radiographic image did not allow accurate assessment of a studied index, this index was excluded from analysis. Decisions concerning the exclusion were made by the project PI (EB) after discussion with Musculoskeletal Radiologists. All these exclusions were then taken into consideration as a potential source of bias.
The collected data were entered in to the electronic data base for further analysis. Data quality control was performed by 2 experts (EB and AN), and disagreements were resolved by discussion.
Following indices were applied to characterize the studied population: Quantitative characteristics were analyzed using number of observations, mean, standard deviation, median, minimum and maximum values[38]. Percentiles were applied for better description of distribution of spinopelvic characteristics before and after operation, and also difference between postoperative vs preoperative values (level of correction)[38,41]. Categorical characteristics were analyzed using percentage[38].
Cumulative number and incidence rate for each of MC for the studied postoperative period was calculated as a rate (0-1) or percentage with 95% confidence limits (95%Cl)[41]. To define the postoperative period with maximum likelihood of occurrence, distribution of latent periods (time between the index operation and diagnosis of MC) were analyzed using percentiles[41]. Risk of postoperative revision/reoperation associated with each type of MC was defined by OR with 95%Cl[41]. The P-value was defined by the χ2-test[41]; if the number of studied events in any of the analyzed subgroups was small (≤ 5), the Fisher-exact test (F) was applied[41]. Analysis of risk factors associated with MC was performed in a few stages. Initially, there were revealed factors that have statistically significant (P ≤ 0.05) association with any type of MC. This analysis was performed using the logistic regression[38]. Then, quantitative characteristics that showed such association were categorized to define an exact range with the most significant risk of MC. The level of risk was assessed by OR (95%Cl), and P-value was defined by the χ2 or the Fisher exact tests[41]. Categorical indices were analyzed to identify a category having the most significant association with the MC. The level of risk was also assessed by OR (95%Cl), and P-value by the χ2 or the Fisher exact tests[41]. Grouping analysis was applied to reveal risk factors that are general for all or a few types of MC; in particular, those that are mainly associated with MC of the spine, and those that are mainly linked with MC of spinal instrumentation. Impact of confounders and combinations of different risk factors was defined by stratification, if it was applicable due to the number of cases[38]. Multiple regression analysis was applied to define prognostic capability of an integrative approach when a few risk factors having significant association with MC are taken into consideration. The JMP®7.0.1 (SAS Institute Inc., United States; http://www.jmp.com) statistical program was used for analysis. The next stage of analysis included assessment of predictive values for each revealed risk factor. The predictive values included sensitivity (Sn), specificity (Sp), positive (+PV) and negative (-PV) predictive values[38]. There was applied following definition for each predictive value taking into consideration context of this study: Sn is the probability of risk factor presence if the MC has been diagnosed during the studied follow-up period; Sp is the probability of risk factor absence if the MC has been not diagnosed during the studied follow-up period; +PV is the probability of diagnosed MC during the studied follow-up period if the risk factor is present; -PV is the probability of absence of diagnosed MC during the studied follow-up period if the risk factor is absent. The Bayesian method was used to generalize the obtained results with previous findings, if it was applicable due to the quality of the previously published data. The previously published data were used as prior odds combining of which with the result of current study provided posterior odds (PO) which combined effects of the previous and the current data[42,43]. If the PO was ≥ 5 or ≤ 0.2, it was considered as a sufficient level of evidence of the generalized effect[42,43]. The statistical analysis was performed by statistician experienced in analysis of biomedical data who is a coauthor this publication (AN).
Initially, 118 patients who underwent 130 osteotomies were identified. Thirty patients and 36 corresponding operations were excluded due to < 2 year postoperative follow-up. Eighty-eight patients who had 94 operations were included; 6 of 88 patients had 2 operations, each was analyzed as a separate case. In total, 94 cases were analyzed. Mean follow-up was 30 mo.
The demographic characteristics of the included cases were: Female 68%, male 32%; mean age, 58.6 (SD, 12.7); mean BMI, 26.6 (SD, 5.6); smoking at the time of operation, 14.9%; ethnicity: Caucasians 87.2%, Hispanic 7.4, other 5.3% (Table 1).
| Demographic characteristic | Subgroup (if applicable) | Measure units | Statistical characteristics | Value |
| Age | NA | Years | n | 94 |
| Median | 59.5 | |||
| Mean | 58.6 | |||
| SD | 12.7 | |||
| Min | 23 | |||
| Max | 82 | |||
| Gender | Male | Subjects | Number (%) | 30 (32) |
| Female | Number (%) | 64 (68) | ||
| Body mass index | NA | Conventional units | Number | 79 |
| Median | 26.6 | |||
| Mean | 27.2 | |||
| SD | 5.6 | |||
| Min | 16.2 | |||
| Max | 43.5 | |||
| Primary diagnosis | Degenerative scoliosis | Subjects | Number (%) | 9 (9.6) |
| Idiopathic scoliosis | Number (%) | 29 (31.1) | ||
| Degenerative kyphosis | Number (%) | 13 (13.9) | ||
| Mixed and other adult spine deformities | Number (%) | 43 (45.4) | ||
| Smoking status | Never | Subjects | Number (%) | 38 (40.4) |
| Former | Number (%) | 37 (39.4) | ||
| Current | Number (%) | 14 (14.9) | ||
| Not specified | Number (%) | 5 (5.3) | ||
| Ethnicity | Caucasians | Subjects | Number (%) | 82 (87.2) |
| Hispanic | Number (%) | 7 (7.4) | ||
| Not specified | Number (%) | 5 (5.3) | ||
| Osteoporotic status | Osteoporosis or osteopenia | Subjects | Number (%) | 29 (30.3) |
The primary diagnosis included: Degenerative scoliosis and/or kyphosis, 23.5%; idiopathic scoliosis, 31.1%; combination of different etiologies of adult spine deformity, 45.4%. Concomitant osteoporosis or osteopenia: 30.3% (Table 1).
The characteristic of the studied (index) operations included: Primary, 21%; revision, 79%; number of levels fused: median 8, minimum 2 and maximum 17; type of osteotomy: SPO, 46%; PSO, 54%; osteotomy location: Lumbar, 62%; thoracic, 21%, thoracolumbar junction, 14%, and sacrum, 3%; transitional segments crossed by instrumentation: cervicothoracic, 2%; thoracolumbar, 26%; lumbosacral, 14%; thoracolumbar and lumbosacral, 51%; fixation to sacrum, 40%; fixation to pelvis, 23%; use of anterior fusion, 38%; number of anterior levels fused: Median, 2; minimum 2 and maximum 6; supplemental anterior fixation, 66%; cement use, 25%; BMP use, 52%; use of individually precontoured posterior rods, 34%; connecting of new instrumentation to previously implanted, 17%; use of domino and/or parallel connectors, 14% (Table 2).
| Characteristics of operation | Subgroup (if applicable) | Measure units | Statistical characteristic | Value |
| Index operation | Primary | Subjects | Number (%) | 20 (21) |
| Reoperation | Number (%) | 74 (79) | ||
| Number of fused levels | NA | Number | Median | 8 |
| Min; max | 2; 17 | |||
| Type of osteotomy | PSO | Subjects | Number (%) | 51 (54) |
| SPO | Number (%) | 43 (46) | ||
| Osteotomy location | Lumbar | Subjects | Number (%) | 58 (62) |
| Thoracic | Number (%) | 20 (21) | ||
| Thoracolumbar junction | Number (%) | 13 (14) | ||
| Sacrum | Number (%) | 3 (3) | ||
| Inter-level junctions crossing by instrumentation | Cervicothoracic | Subjects | Number (%) | 2 (2) |
| Thoracolumbar | Number (%) | 24 (26) | ||
| Lumbosacral | Number (%) | 13 (14) | ||
| Thoracolumbar and lumbosacral | Number (%) | 48 (51) | ||
| No | Number (%) | 7 (7) | ||
| Fixation to sacrum (not pelvis) | NA | Subjects | Number (%) | 38 (40) |
| Fixation to sacrum and/or pelvis | NA | Subjects | Number (%) | 59 (63) |
| Use of anterior fusion | NA | Subjects | Number (%) | 36 (38) |
| Number of anterior levels fused | NA | Subjects | Median | 2 |
| Min; max | 1; 6 | |||
| Supplemental anterior support/fixation by implant or instrumentation | NA | Subjects | Number (%) | 62 (66) |
| Use of cement | NA | Subjects | Number (%) | 23 (25) |
| Use of bone morphogenetic protein | NA | Subjects | Number (%) | 49 (52) |
| Use of individually precontoured posterior rods | Precontoured | Subjects | Number (%) | 32 (34) |
| In situ contouring | Subjects | Number (%) | 62 (66) | |
| Connecting to previously implanted instrumentation | NA | Subjects | Number (%) | 16 (17) |
| Use of Domino and/or parallel connectors | 2 | Subjects | Number (%) | 2 (2) |
| 1 | Number (%) | 11 (12) | ||
| 0 | Number (%) | 81 (86) |
The number of high quality images adequate for obtaining spinopelvic parameters was limited, but was enough to reach statistically significant results. Two independent evaluations of the studied radiographic indices showed good agreement by the Kappa test, 0.85 (SE, 0.09), P = 0.08.
Preoperative and postoperative characteristics of sagittal spinopelvic alignment were highly heterogeneous having distribution close to the normal (bell shaped curve) (Table 3). In particular, preoperative SVA ranged from 0 to 203 mm; 70% of patients had anterior sagittal imbalance (SVA > 50 mm, Table 3). After operative treatment the mean SVA decreased from 73.9 mm to 41.7 mm (P < 0.001) with the percentage of patients with the sagittal imbalance decreasing to 35%. However, variability remained high with values ranging from -66 mm to 167 mm. The absolute difference between postoperative and preoperative values ranged from 0 to 182 mm (Table 3).
| Spinopelvic characteristic, units | Subgroup | n | Characteristics of distribution | ||||||||
| Percentiles | Mean | SD | |||||||||
| Min | 10 | 25 | 50 | 75 | 90 | Max | |||||
| Sagittal vertical axis, mm | Preop | 94 | 0 | 15 | 33 | 74 | 105 | 128 | 203 | 73.9 | 42.4 |
| Postop | 61 | -66 | 0.2 | 14 | 40 | 60 | 94 | 167 | 41.7 | 38.4 | |
| Postop-Preop | 61 | 73 | 34 | -5 | -30 | -59 | -96 | -182 | -33.1 | 47.8 | |
| Postop-Preop (abs) | 61 | 0 | 10 | 20 | 38 | 60 | 96 | 182 | 46 | 35.7 | |
| Lumbar lordosis, L1-S1, degree | Preop | 77 | 4 | 8 | 19 | 36 | 45 | 56 | 99 | 34.4 | 19.4 |
| Postop | 90 | 3 | 32 | 43 | 52 | 61 | 71 | 97 | 51.3 | 16.6 | |
| Postop-Preop | 74 | -37 | -6 | 4 | 16 | 30 | 38 | 50 | 16.1 | 17.4 | |
| Postop-Preop (abs) | 74 | 1 | 3 | 9 | 17 | 31 | 37 | 50 | 19.7 | 13.2 | |
| Thoracic kyphosis, T1-T12, degree | Preop | 64 | -3 | 11 | 23 | 39 | 54 | 64 | 109 | 39.4 | 21.9 |
| Postop | 81 | 9 | 18 | 32 | 44 | 51 | 58 | 75 | 41.9 | 14.7 | |
| Postop-Preop | 62 | -41 | -19 | -10 | 1 | 13 | 25 | 34 | 1.6 | 15.9 | |
| Postop-Preop (abs) | 62 | 0 | 1 | 2 | 12 | 18 | 29 | 41 | 12 | 10.3 | |
| Pelvic tilt, degree | Preop | 68 | 8 | 13 | 19 | 27 | 35 | 43 | 48 | 27.8 | 10.4 |
| Postop | 78 | -4 | 9 | 13 | 20 | 27 | 33 | 51 | 20.2 | 9.7 | |
| Postop-Preop | 61 | 10 | 5 | -2 | -8 | -15 | -19 | -29 | -7.9 | 9.1 | |
| Postop-Preop (abs) | 61 | 0 | 2 | 5 | 8 | 15 | 19 | 29 | 9.9 | 6.7 | |
| Pelvic incidence – lumbar lordosis mismatch, degree | Preop | 68 | -43 | -10 | 9 | 23 | 37 | 47 | 66 | 20.9 | 21.6 |
| Postop | 78 | -41 | -17 | -7 | 2 | 13 | 28 | 41 | 3.8 | 15.6 | |
| Postop-Preop | 61 | 25 | 4 | -2 | -18 | -31 | -37 | -48 | -16.2 | 16.6 | |
| Postop-Preop (abs) | 62 | 0 | 3 | 9 | 18 | 31 | 37 | 48 | 19.2 | 13.2 | |
Preoperative LL ranged from 4° to 99° with mean value 34.4°. After surgical treatment the mean value increased to 51.3° (P < 0.001), but variability remained high (Table 3). Absolute difference between postoperative and preoperative LL values varied from 1° to 50° (Table 3).
The mean preoperative TK was 39.4°, and extreme values ranged from -3° to 109°. While the mean value did not change significantly after operative treatment (41.9°), the absolute difference between postoperative and preoperative values varied from 0° to 41° with the mean value 12° suggesting significant (P < 0.001) reciprocal postoperative change (Table 3).
Preoperative PT ranged from 8° to 40° with a mean value of 27.8°. After surgery the mean decreased to 20.2 (P < 0.001), but variability remained high with extreme values from -4° to 51°. The absolute difference between postoperative and preoperative values ranged from 0° to 29° with a mean of 9.9° (Table 3).
Preoperative PI-LL ranged from -43° to 66° with the mean 20.9°. The mean decreased postoperatively to 3.8° (P < 0.001), but the range remained approximately the same. The absolute difference between postoperative and preoperative values ranged from 0° to 48° with the mean, 19.2° (Table 3).
A fall after surgery was observed in 15% (95%Cl: 11.3; 18.7) of cases. Postoperative pseudoarthrosis was revealed in 10.6% (95%Cl: 7.4; 13.6) of cases. One MC had 27.6% (95%Cl: 23.0; 32.2), and multiple MC (from 2 to 4) had 16% (95%Cl: 12.2; 19.8) of the patients. Additional postoperative treatment was required in 57.5% of cases, including 42.5% (95%Cl: 37.4; 47.6) requiring revision surgery, and 15% (95%Cl: 11.3; 18.7) conservative treatment in only (Table 4).
| Index | Number of cases | Rate, % (95%Cl: min; max) |
| Fall after operation before mechanical complication(s) | 14 | 15% (11.3; 18.7) |
| Postoperative pseudarthrosis | 10 | 10.6% (7.4; 13.6) |
| Cases with 1 mechanical complication | 26 | 27.6% (23.0; 32.2) |
| Cases with a few (2-4) mechanical complications | 15 | 16.0% (12.2; 19.8) |
| Additional surgical treatment (revision/reoperation) | 40 | 42.5% (37.4; 47.6) |
| Additional conservative treatment | 14 | 15.0% (11.3; 18.7) |
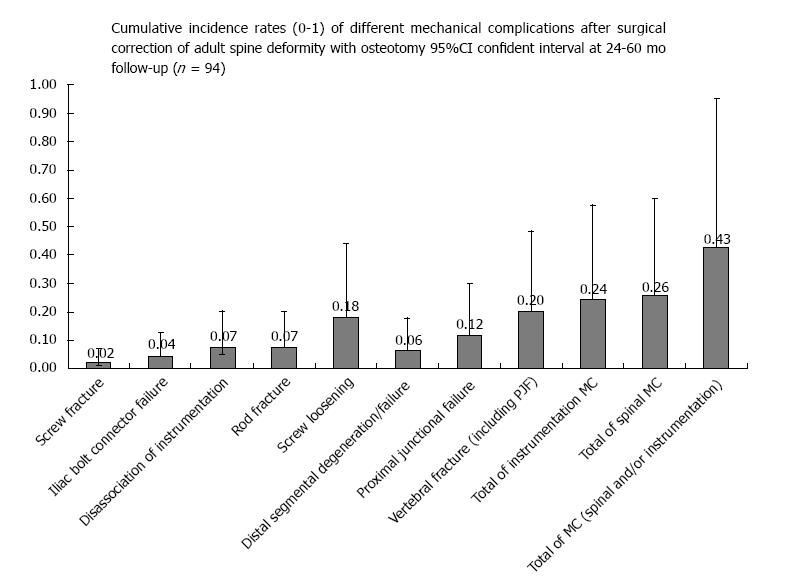
The total incidence of cases with MC was 43.6% (95%Cl: 33.4; 53.8%). MC of the spine occurred in 25.5% (95%Cl: 16.5; 34.5); and MC of the instrumentation in 25.5% (95%Cl: 16.5; 34.5, Table 5). Cases with MC of the spine included: VF in 20.2% (95%Cl: 12.0; 28.4), PJF in 11.7% (95%Cl: 5.2; 18.2), and DSF in 6.4% (95.5Cl: 1.4; 11.4%, Table 5). Cases with MC of the instrumentation included: SL in 18.1% (95%Cl: 14.1; 22.1), fracture of the screw in 2.1% (95%Cl: 0; 4.9), RF in 7.4% (95%Cl: 4.7; 10.1), IBCF in 4.3% (95%Cl: 2.2; 6.5), and DI in 7.4% (95%Cl: 4.7; 10.1) (Figure 6 and Table 5).
| Mechanical complication | n1 | Association with revision/reoperation after index operation | ||
| n2 | OR (95%Cl) | P value | ||
| Total (failure of spine and/or instrumentation) | 41 | 32 | 20.0 (6.9; 57.4) | < 0.0001 |
| Failure of spine (total) | 24 | 21 | 18.8 (5.0; 70.3) | < 0.0001 |
| Vertebral fracture (total) | 19 | 17 | 19.2 (4.1; 90.1) | < 0.0001 |
| PJF | 11 | 11 | > 19.0 | < 0.0001 |
| DSF | 6 | 5 | 7.6 (0.8; 67.6) | 0.033 |
| Instrumentation failure (total) | 24 | 18 | 6.6 (2.3; 18.8) | 0.001 |
| Screw loose | 17 | 12 | 4.2 (1.3;13.2) | 0.014 |
| Screw fracture | 2 | 2 | NA | 0.161 |
| Rod fracture | 7 | 4 | 1.9 (0.4; 8.9) | 0.453 |
| Iliac bolt connector (loose and/or fracture) | 4 | 3 | 5.7 (0.6; 53.4) | 0.308 |
| Disconnection of instrumentation | 7 | 6 | 9.4 (1.1; 81.1) | 0.022 |
An association between MC and secondary surgical treatment was strong, OR = 20 (95%Cl: 6.9; 57.4, P < 0.001) with 78% of cases of MC (32 of 41) leading to revision surgery. This association was the most significant (P < 0.04) in cases with MC of the spine, in particular: VF, PJF and DSF. It was also significant in MC of the instrumentation such as: SL and DI (Table 5).
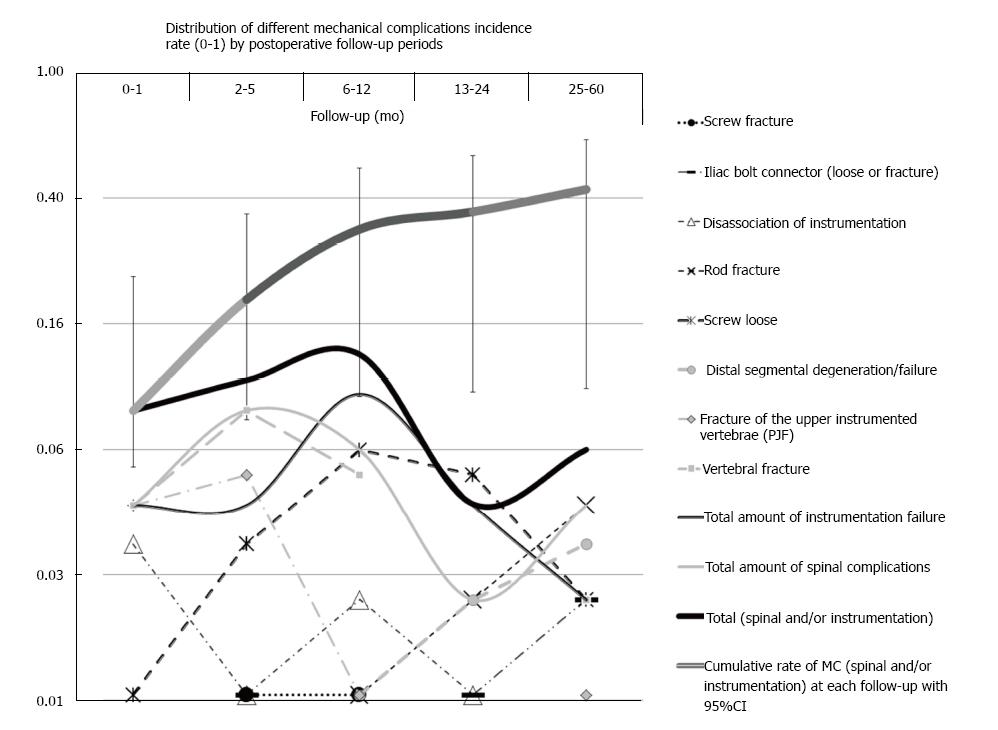
Majority of MC (70%) were diagnosed during the 1st postoperative year (Figure 7). The shortest latent period had PJF, VF, SL (specifically screw fracture), and DI (≥ 70% of these cases was revealed during 1st year). Longer latent periods (> 1 year in majority of cases) were seen in MC such as DSF, SL, RF, IBCF (Figure 7).
The following factors had significant association with MC (all types): Preoperative SVA > 110 mm, OR = 4.5 (95%Cl: 1.3; 15.4), P = 0.011; postoperative SVA > 74 mm, OR = 5.4 (95%Cl: 1.4; 21.1), P = 0.014; preoperative LL < 20o, OR = 5.5 (95%Cl: 1.8; 16.9), P = 0.002; postoperative change of LL > 34°, OR = 4.7 (95%Cl: 1.2; 18.0), P = 0.028; postoperative change of PI-LL > 34°, OR = 6.4 (95%Cl: 1.2; 33.2), P = 0.033; type of osteotomy, SPO vs PSO, OR = 0.42 (95%Cl: 0.18; 1.0), P = 0.045; fixation to sacrum with or without fixation to pelvic after PSO if number of fused levels > 4, OR = 3.6 (95%Cl: 0.92; 13.9), P = 0.056; postoperative pseudarthrosis, OR = 14.6 (95%Cl: 1.8; 120.9), P = 0.002 (Table 6). However, in spite of statistical significance, prediction capacity of these factors was limited, in particular, majority of them had low Sn (< 40%) (Table 6). To take into consideration all factors listed above, an integral index was obtained by multiple regression analysis using equation (1) described below. With this equation risk of MC ranged from 0 to 1. Analysis showed that the index values ≥ 0.46 have the highest association with MC (OR = 31.7, 95%Cl: 6.7; 149.3, P < 0.0001, Table 6). Predictive capacity of this integral index was in general higher than that of single characteristics, but did not exceed moderate level: Sn, 79%; Sp, 89%; +PV, 86%, and -PV, 83%.
| Risk factor(s) | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | |||
| Preoperative SVA > 110 mm | 4.5 (1.3; 15.4) | 0.011 | 0.27 | 0.92 | 0.73 | 0.62 |
| Postoperative SVA > 75 mm | 5.4 (1.4; 21.1) | 0.014 | 0.24 | 0.94 | 0.77 | 0.62 |
| Preoperative LL< 20° | 5.5 (1.8; 16.9) | 0.002 | 0.37 | 0.91 | 0.75 | 0.65 |
| Postoperative change of LL > 34° (absolute values) | 4.7 (1.2; 18.0) | 0.028 | 0.22 | 0.94 | 0.75 | 0.61 |
| Postoperative change of PI-LL > 34° (absolute values) | 6.4 (1.2; 33.2) | 0.033 | 0.29 | 0.94 | 0.80 | 0.62 |
| Type of osteotomy SPO vs PSO | 0.42 (0.18; 1.0) | 0.045 | 0.34 | 0.45 | 0.33 | 0.47 |
| Fall after operation before mechanical complication | 6.1 (1.6; 23.7) | 0.007 | 0.27 | 0.94 | 0.79 | 0.63 |
| Fixation to sacrum or pelvic if number of fused levels > 4 | 2.4 (0.9; 6.4) | 0.068 | 0.74 | 0.46 | 0.53 | 0.68 |
| Fixation to sacrum or pelvic if number of fused levels > 4 with PSO | 3.6 (0.92; 13.9) | 0.053 | 0.78 | 0.50 | 0.67 | 0.64 |
| Postoperative pseudarthrosis | 14.6 (1.8; 120.9) | 0.002 | 0.22 | 0.98 | 0.90 | 0.62 |
| Integral index based on parameters presented above by results of multiple regression modeling (1) ≥ 0.46 | 31.7 (6.7; 149.3) | < 0.001 | 0.79 | 0.89 | 0.86 | 0.83 |
y = 0.84 + X1 + X2 + X3 + X4 + X5 + X6 + X7 + X8 + X9 (1)
Where: y is risk of MC ranged from 0 to 1; X1 is preoperative LL: < 20° match to 0.22, and ≥ 20° match to (-0.22); X2 is fall after surgery, but before MC: “yes”, match to 0.15, and “no”, match to (-0.15); X3 is fixation to sacrum or pelvic: “yes”, match to 0.07, and “no”, match to (-0.07); X4 is preoperative SVA: ≥ 110 mm match to 0.04, and < 110 mm match to (-0.04); X5 is postoperative SVA: ≥ 75 mm match to 0.14, and < 75 mm match to (-0.14); X6 is postoperative change of LL: ≥ 35° match to 0.07, and < 35° match to (-0.07); X7 is postoperative change of PI-LL: ≥ 35° match to 0.001, and < 35° match to (-0.001); X8 is type of osteotomy: PSO match to 0.05, and SPO match to (-0.05); and X9 is presence of postoperative pseudarthrosis: “yes”, match to 0.17, and “no” match to (-0.17).
Factors that have significant association with MC of the spine include: Postoperative SVA > 107 mm, OR = 11.3 (95%Cl: 1.1; 118.1), P = 0.043; type of osteotomy (SPO vs PSO), OR = 0.39 (95%Cl: 0.1; 1.1), P = 0.046; fall after surgery, OR = 5.3 (95%Cl: 1.6; 17.5), P = 0.006; postoperative change of TK > 25° in absolute values, OR = 4.8 (95%Cl: 1.1; 20.8), P = 0.041; postoperative change of PT ≥ 9° in absolute values, OR = 3.3 (95%Cl: 0.9; 11.1), P = 0.049; postoperative change of PI-LL ≥ 35° in absolute values, OR = 3.8 (95%Cl: 0.9; 15.6), P = 0.059. The integral characteristic ≥ 0.46 showed higher association, OR = 14.0 (95%Cl: 27; 73.6), P < 0.001 (Table 7). Nevertheless, all these indices had limited predictive values, in particular, low Sn and +PV (Table 7). Prognostic capability of the integrative characteristic was somewhat better, but +PV was only 50% (Table 7).
| Risk factor(s) | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | |||
| Postoperative SVA > 106 mm | 11.3 (1.1; 118.1) | 0.043 | 0.20 | 0.98 | 0.75 | 0.79 |
| Type of osteotomy: SPO vs PSO | 0.39 (0.1;1.1) | 0.046 | 0.29 | 0.49 | 0.16 | 0.67 |
| Fall after operation before mechanical complication | 5.3 (1.6; 17.5) | 0.006 | 0.33 | 0.91 | 0.57 | 0.80 |
| Postoperative change of TK > 25° (absolute values) | 4.8 (1.1; 20.8) | 0.041 | 0.31 | 0.91 | 0.56 | 0.79 |
| Postoperative change of PT > 8° (absolute values) | 3.3 (0.9; 11.1) | 0.045 | 0.69 | 0.60 | 0.38 | 0.84 |
| Postoperative change of PI-LL > 34° (absolute values) | 3.8 (0.9; 15.6) | 0.065 | 0.31 | 0.89 | 0.50 | 0.79 |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 14 (2.7; 73.6) | < 0.001 | 0.85 | 0.72 | 0.50 | 0.93 |
Factors having significant association with VF were: smoking, OR = 5.7 (95%Cl: 1.7; 19.1), P = 0.008; SPO vs PSO if osteotomy, OR = 0.25 (95%Cl: 0.1; 0.8), P = 0.02; and fall after operation, OR = 4.3 (95%Cl: 1.3; 14.5), P = 0.024 (Table 8). However, Sn and +PV of these variables was < 50% (Table 8). The integrative index had twice higher association, OR = 11.7 (96%Cl: 2.2; 61.5), P < 0.001, as well as general prognostic capacity, but +PV was approximately the same (Table 8). Those factors having significant association with PJF included: Smoking, OR = 4.2 (95%Cl: 1.0; 16.8), P = 0.05; SPO vs PSO of osteotomy, OR = 0.23 (95%Cl: 0.05; 1.1), P = 0.041; a fall after the operation, OR = 4.2 (95%Cl: 1.0; 16.8), P = 0.05; osteoporosis or osteopenia in cases treated with a PSO having > 5 fused levels, OR = 10.4 (95%Cl: 0.8; 137.8), P = 0.039; reoperation vs primary operation, OR = 20.1 (95%Cl: 2.5; 163.6), P < 0.001 (Table 8). However, all these characteristics had low +PV (< 30%) (Table 8). The integrative index did not show significant association with PJF, P = 0.167 (Table 8).
| Mechanical complication of spine | Risk factors | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | ||||
| Vertebral fracture | Current smoking | 5.7 (1.7; 19.1) | 0.008 | 0.37 | 0.91 | 0.50 | 0.85 |
| Type of osteotomy: SPO vs PSO | 0.25 (0.1; 0.8) | 0.020 | 0.21 | 0.48 | 0.09 | 0.71 | |
| Fall after operation before mechanical complication | 4.3 (1.3; 14.5) | 0.024 | 0.32 | 0.89 | 0.43 | 0.84 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 11.7 (2.2;61.5) | < 0.001 | 0.83 | 0.70 | 0.45 | 0.93 | |
| Proximal junctional failure | Current smoking | 4.2 (1.0; 016.8) | 0.055 | 0.36 | 0.88 | 0.29 | 0.91 |
| Osteoporosis/osteopenia | 3.19 (0.9; 11.3) | 0.075 | 0.55 | 0.72 | 0.21 | 0.92 | |
| PSO and > 5 levels fused in osteoporosis/osteopenia | 10.4 (0.8; 137.8) | 0.039 | 1.00 | 0.84 | 0.29 | 1.00 | |
| Fall after operation before mechanical complication | 4.2 (1.0; 16.8) | 0.055 | 0.36 | 0.88 | 0.29 | 0.91 | |
| Reoperation vs primary operation | 20.1 (2.5; 163.6) | < 0.001 | 0.92 | 0.65 | 0.28 | 0.98 | |
| Type of osteotomy: SPO vs PSO | 0.23 (0.05; 1.1) | 0.048 | 0.18 | 0.51 | 0.05 | 0.82 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 4.6 (0.4; 47.3) | 0.167 | 0.75 | 0.60 | 0.14 | 0.97 | |
| Distal segmental degeneration/ failure | Preoperative LL ≤ 20° | 20.9 (2.3; 190.6) | 0.002 | 0.83 | 0.81 | 0.23 | 0.99 |
| Thoracolumbar crossing junction | 18.6 (2.0; 164.9) | 0.004 | 0.83 | 0.78 | 0.21 | 0.99 | |
| Fixation to sacrum or pelvic | 0.08 (0.01; 0.73) | 0.001 | 0.14 | 0.33 | 0.02 | 0.83 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 2.9 (0.1; 89.1) | 0.186 | 0.67 | 0.59 | 0.05 | 0.98 | |
Factors having significant association with DSF included: preoperative LL ≤ 20°, OR = 20.9 (95%Cl: 2.3; 190.6), P = 0.002; crossing the thoracolumbar junction, OR = 18.6 (95%Cl: 2.0; 164.9), P = 0.004; fixation to the sacrum or pelvis, OR = 0.08 (95%Cl: 0.01; 0.73), P = 0.001 (Table 8). However, +PV of these characteristics was low (< 25%) (Table 8). The integrative index did not show significant association with DSF, P = 0.167 (Table 8).
Factors having significant association with MC of instrumentation were: Preoperative SVA ≥ 100 mm, OR = 4.1 (95%Cl: 1.5; 11.3), P = 0.007; postoperative change of SVA > 76 mm in absolute value, OR = 4.4 (95%Cl: 1.1; 18.0), P = 0.041; postoperative SVA < 50 mm in cases with preoperative SVA > 110 mm, OR = 9.3 (95%Cl: 1.4; 63.2), P = 0.025; crossing the thoracolumbar and/or lumbosacral junction(s), OR = 6.5 (95%Cl:1.4; 30.2), P = 0.0.6; fixation to the sacrum and/or pelvis, OR = 4.0 (95%Cl: 1.2; 12.1), P = 0.025; maximum rod contouring angle ≥ 60°, OR = 4.4 (95%Cl: 1.2; 16.1), P = 0.025; the use of rods individually precontoured by the manufacturer, OR = 0.35 (95%Cl: 0.1;1.3), P = 0.05; use of more than 1 domino and/or parallel connectors, OR = 6.3 (95%Cl: 0.5; 72.6), P = 0.06; preoperative LL ranging from 48° to 60°, OR = 0.15 (95%Cl: 0.01; 1.3), P = 0.05; postoperative pseudarthrosis, OR = 9.2 (95%Cl: 2.1; 39.3), P = 0.002, and the integrative index ≥ 0.46, OR = 7.8 (95%Cl: 2.0; 29.9), P = 0.002 (Table 9). In spite of the revealed statistically significant association the predictive capacity of all these characteristics was limited, in particular, +PV did not exceed 70% (Table 9).
| Factors | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | |||
| Preoperative SVA ≥ 100 mm | 4.1 (1.5; 11.3) | 0.007 | 0.46 | 0.83 | 0.48 | 0.82 |
| Postoperative change of SVA > 76 mm (absolute values) | 4.4 (1.1; 18.0) | 0.041 | 0.32 | 0.90 | 0.60 | 0.75 |
| Postoperative SVA < 50 mm, if preoperative SVA≥ 110 mm | 9.3 (1.4; 63.2) | 0.025 | 0.67 | 0.82 | 0.40 | 0.93 |
| Preoperative LL 48°-60° | 0.15 (0.01; 1.3) | 0.043 | 0.05 | 0.76 | 0.07 | 0.67 |
| Thoracolumbar and/or lumbosacral crossing junction(s) | 6.5 (1.4; 30.2) | 0.006 | 0.92 | 0.37 | 0.36 | 0.92 |
| Fixation to sacrum and/or pelvic | 4.0 (1.2; 12.8) | 0.026 | 0.83 | 0.44 | 0.34 | 0.89 |
| Maximum rod contouring angle > 60° | 4.4 (1.2; 16.1) | 0.025 | 0.62 | 0.73 | 0.40 | 0.87 |
| Precontoured posterior rods vs in situ contouring | 0.35 (0.1; 1.3) | 0.050 | 0.38 | 0.36 | 0.16 | 0.65 |
| Domino and/or parallel connectors number > 1 | 6.3 (0.5; 72.6) | 0.063 | 0.08 | 0.99 | 0.67 | 0.76 |
| Postoperative pseudarthrosis | 9.2 (2.1; 39.3) | 0.002 | 0.29 | 0.96 | 0.70 | 0.80 |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 7.8 (2.0; 29.9) | 0.002 | 0.75 | 0.72 | 0.55 | 0.87 |
The factors having significant association with SL included: Preoperative SVA > 100 mm, OR = 5.1 (95%Cl: 1.6; 15.4), P = 0.005; postoperative change of SVA in absolute value > 76 mm, OR = 5.4 (95%Cl: 1.3; 22.9), P = 0.026; postoperative SVA < 50 mm in cases with preoperative SVA > 110 mm, OR = 20.6 (95%Cl: 2.6; 163.8), P = 0.004; fixation to sacrum and/or pelvis, OR = 2.5 (95%Cl: 0.8; 8.3), P = 0.103; preoperative LL < 34°, OR = 3.4 (95%Cl: 1.0; 10.9), P = 0.034; postoperative pseudoarthrosis, OR = 9.9 (95%Cl: 2.4; 40.0), P = 0.001; rod fracture, OR = 15.6 (95%Cl: 2.7; 89.8), P = 0.001; and the integrative index ≥ 0.46, OR = 29.0 (95%Cl: 3.3; 251.9), P < 0.001 (Table 10). The predictive capability of all these characteristics was limited, +PV ≤ 71% (Table 10).
| Instrumentation failure | Risk factors | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | ||||
| Screw loosening | Preoperative SVA ≥ 100 mm | 5.1 (1.6; 15.4) | 0.005 | 0.53 | 0.82 | 0.39 | 0.89 |
| Postoperative change of SVA > 76 mm (absolute values) | 5.4 (1.3; 22.9) | 0.026 | 0.38 | 0.90 | 0.50 | 0.84 | |
| Postoperative SVA < 50 mm, if preoperative SVA ≥ 110 mm | 20.6 (2.6; 163.8) | 0.004 | 0.67 | 0.91 | 0.57 | 0.94 | |
| Preoperative LL < 34° | 3.4 (1.0; 10.9) | 0.034 | 0.69 | 0.61 | 0.31 | 0.88 | |
| Fixation to sacrum and/or pelvic | 2.5 (0.8; 8.3) | 0.103 | 0.78 | 0.42 | 0.24 | 0.89 | |
| Postoperative pseudarthrosis | 9.9 (2.4; 40.0) | 0.001 | 0.35 | 0.95 | 0.60 | 0.87 | |
| Rod fracture | 15.6 (2.7; 89.8) | 0.001 | 0.29 | 0.97 | 0.71 | 0.86 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 29.0 (3.3;251.9) | < 0.001 | 0.92 | 0.73 | 0.50 | 0.97 | |
| Rod fracture | Preoperative SVA ≥ 100 mm | 9.6 (1.7; 53.5) | 0.008 | 0.71 | 0.79 | 0.22 | 0.97 |
| Postoperative change of SVA > 76 mm (absolute values) | 11.3 (2.0; 63.3) | 0.007 | 0.57 | 0.89 | 0.40 | 0.94 | |
| Postoperative SVA < 50 mm, if preoperative SVA ≥ 110 mm | 33.0 (2.6; 424.1) | 0.002 | 0.50 | 0.97 | 0.75 | 0.92 | |
| Preoperative LL < 20° | 4.9 (1.0; 24.3) | 0.050 | 0.57 | 0.79 | 0.21 | 0.95 | |
| Postoperative change of LL ≥ 30° | 7.4 (1.3; 41.5) | 0.022 | 0.71 | 0.75 | 0.23 | 0.96 | |
| Postoperative pseudarthrosis | 8.6 (1.6; 46.2) | 0.019 | 0.43 | 0.92 | 0.30 | 0.95 | |
| Domino and/or parallel connectors | 5.8 (1.1; 29.6) | 0.052 | 0.43 | 0.89 | 0.23 | 0.95 | |
| Sagittal rod contouring angle > 56° | 9.8 (1.1; 85.2) | 0.019 | 0.86 | 0.62 | 0.15 | 0.98 | |
| Number of crossing junctions > 1 | 5.6 (0.7; 48.5) | 0.087 | 0.86 | 0.48 | 0.12 | 0.98 | |
| Connecting to previously implanted instrumentation | 8.3 (1.7; 41.9) | 0.014 | 0.57 | 0.86 | 0.25 | 0.96 | |
| Iliac bolt connector loose and/or fracture | 17 (1.9;147.0) | 0.026 | 0.67 | 0.89 | 0.50 | 0.94 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 6.9 (0.7; 66.5) | 0.027 | 0.80 | 0.63 | 0.18 | 0.97 | |
The factors having significant association with RF were: preoperative SVA > 100 mm, OR = 9.6 (95%Cl: 1.7; 53.5), P = 0.008; postoperative change of SVA absolute value > 76 mm, OR = 11.3 (95%Cl: 2.0; 63.3), P = 0.007; postoperative SVA < 50 mm in cases with preoperative SVA > 110 mm, OR = 33.0 (95%Cl: 2.6; 424.1), P = 0.002; preoperative LL < 20o, OR = 4.9 (95%Cl: 1.0; 24.3), P = 0.05; postoperative change of absolute values in LL ≥ 30°, OR = 7.4 (95%Cl: 1.3; 41.5), P = 0.022; postoperative pseudoarthrosis, OR = 8.6 (95%Cl: 1.6; 46.2), P = 0.019; use of domino and/or parallel connectors, OR = 5.8 (95%Cl: 1.1; 29.6), P = 0.052; sagittal rod contouring angle > 56°, OR = 9.8 (95%Cl: 1.1; 85.2), P = 0.019; connecting to previously placed instrumentation, OR = 8.3 (95%Cl: 1.7; 41.9), P = 0.014; iliac bolt connector loose and/or fracture, OR = 17.0 (95%Cl: 1.9; 147.0), P = 0.026; the integrative index ≥ 0.46, OR = 6.9 (95%Cl: 0.7; 66.5), P = 0.027 (Table 10). The predictive value of these characteristics was limited, in particular, +PV ranged from 12% to 75% (Table 10).
The factors having significant association with IBCF included: Preoperative SVA > 100 mm, OR = 24.0 (95%Cl: 1.6; 356), P = 0.02; and postoperative change in absolute value of SVA > 76 mm, OR = 7.4 (95%Cl: 0.7; 81.4) > 8, P = 0.029. The integrative index did not show significant association with this type of MC (Table 11). The predictive capability was limited, +PV of these characteristics did not exceed 60% (Table 11).
| Instrumentation failure | Risk factors | OR (95%Cl: min; max) | P value | Predictive values | |||
| Sn | Sp | +PV | -PV | ||||
| Iliac bolt connector loosening/fracture | Preoperative SVA ≥ 100 mm | 24.0 (1.6; 356.0) | 0.021 | 0.75 | 0.89 | 0.60 | 0.94 |
| Postoperative change of SVA > 76 mm (absolute values) | 7.4 (0.7;81.4) | 0.029 | 0.80 | 0.65 | 0.40 | 0.92 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 4.69 (0.4; 47.3) | 0.069 | 0.75 | 0.60 | 0.14 | 0.97 | |
| Disassociation of instrumentation | Type of osteotomy, SPO vs PSO | 0.15 (0.02; 1.3) | 0.050 | 0.13 | 0.51 | 0.02 | 0.86 |
| Lumbar osteotomy | 7.4 (0.7; 81.4) | 0.029 | 0.80 | 0.65 | 0.40 | 0.92 | |
| Fixation to sacrum or pelvic | 4.8 (0.6; 40.8) | 0.039 | 0.88 | 0.41 | 0.12 | 0.97 | |
| Integral index by multiple regression modeling (1) ≥ 0.46 | 4.5 (0.4; 47.4) | 0.069 | 0.75 | 0.60 | 0.14 | 0.97 | |
Finally, factors having significant association with DI were: SPO vs PSO, if osteotomy applied, OR = 0.15 (95%Cl: 0.02; 1.3), P = 0.050; osteotomy of the lumbar spine, OR = 7.4 (95%Cl: 0.7; 81.4), P = 0.029; and fixation to sacrum and/or pelvis, OR = 4.80.2, P = 0.04, the integrative index did not show significant association with this type of MC, Table 11. The predictive capability was limited; +PV of these characteristics was ≤ 40% (Table 11).
To evaluate the postoperative MC and associated risk factors after surgical correction of ASD with an osteotomy, a case-series of 94 consecutive operations performed in 88 patients were studied. The incidence of postoperative return to the operating room after the index surgery was 42.5%. The cumulative incidence of MC was 43.6%. The incidence of MC of the spine and MC of instrumentation were approximately similar, 25%. Of those, 16% of cases had multiple MC. The most typical MC of the spine were VF (20.2%), PJF (11.7%), and less common DSF (6.4%) (Figure 6 and Table 5). The most typical MC of instrumentation was SL (18.1%), other MC of instrumentation had incidence ranging from 2% to 7.4% (Figure 6 and Table 5).
Around 70% of all MC were diagnosed during the 1st postoperative year. The majority of MC with the shortest latent period were linked with failure of the spine (VF and PJF, where 70% of cases were revealed during 8 mo of follow-up) (Figure 7). Approximately the same latent period also showed higher rates of SL (specifically screw fracture) and DI (Figure 7). It is necessary to note that 2 cases of screw fracture observed in the current study were accompanied with fractures of the pedicle. The MC with a longer latent period (70% of cases were revealed at ≥ 19 mo of follow-up) tended to be linked with instrumentation failure. This included RF, and IBCF (Figure 7). As expected, DSF had a longer latent period closer to that of IBCF (Figure 7).
The MC had significant association with postoperative return to the operating room (OR = 20.0). The strongest association showed MC of the spine, in particular: VF, PJF (OR ≥ 19.0), and DSF (OR = 7.6). Among MC of instrumentation, significant association with secondary surgical treatment showed cases of SL (OR = 4.2) and DI (OR = 9.4). It may be explained by the fact that MC of the spine occurred early, preceding solid fusion, and provoked the corresponding severe clinical symptoms. Some of the MC of instrumentation, in particular RF and IBCF occurred later. There were seen even after the development of solid intervertebral fusion. They were less likely to lead to spinal instability and clinical symptoms requiring surgical treatment.
The revealed characteristics that have significant association with increased risk of MC may be classified into subgroups. First, indices linked with severity of the preoperative sagittal imbalance including: SVA > 100o; and LL< 34o. Second, preoperative comorbidities such as: smoking, and osteoporosis/osteopenia (specifically, in cases after PSO with more than 5 levels fused). Third, postoperative events such as: a fall and pseudoarthrosis. The fourth subgroup reflects insufficient correction of the sagittal imbalance (postoperative SVA > 75 mm). The fifth group includes indices linked with over-correction of the sagittal imbalance and spinopelvic alignment or reciprocal changes including: Postoperative SVA < 50 mm, if preoperative SVA ≥ 110 mm; postoperative changes in absolute values for SVA > 76 mm, TK > 25°, LL > 29°, PI-LL > 35°, and PT > 9°. Also, characteristics of the index operation and the surgical technique were associated with MC: Revision surgery, type of osteotomy (PSO), lumbar location of the osteotomy, crossing transitional spinal segments (thoracolumbar, lumbosacral, and > 1 junction crossed). The seventh group included characteristics of the instrumentation and the fusion construct including: Sagittal rod contouring > 60°, fixation to the sacrum or pelvis (specifically after PSO with > 5 level fused), use of dominos and/or parallel connectors, and connecting to the previously implanted instrumentation. Finally, the other device failures, in particular, RF associated with SL.
Off note, some factors had significant association with low risk of MC or had multiple effects. This was seen in particular with cases having preoperative LL ranging between 47° and 61° (showed lower risk of MC of instrumentation), fixation to the sacrum with or without fixation to pelvis increased risk of SL, but expected decreased risk of DSF, and use of rods individually precontoured by manufacturer decreased risk of MC of instrumentation.
Unlike previous investigators we regarded postoperative pseudarthrosis as a risk factor rather than a MC. This showed that pseudarthrosis was significantly association with MC of instrumentation, in particular SL and RF. This association may reflect progression of postoperative instability, which increases strain at the fused spinal segments preventing ossification of the callus and contributing to the risk of instrumentation failure.
The main results of the current study correspond with previous findings. The results revealed the incidence of pseudarthrosis, revision/reoperation, and severe PJF values very close to those previously reported[11,16,25]. The postoperative period of occurrence for PJF and RF also corresponded with the previously published time-frames[27,44]. It was demonstrated by Charosky et al[11] (2012) that the use of a PSO is associated with higher risk of MC. This corresponds with the results of the current study. However, an additional analysis has shown that a PSO (as expected) is more often applied in cases with severe preoperative sagittal imbalance (SVA ≥ 100 mm). This high starting SVA is also a risk factor of MC, making the argument somewhat circular. Stratification demonstrated that in cases with a preoperative SVA < 100 mm, a PSO showed higher risk of MC than SPO (OR = 2.3; P = 0.1), while in the cases with the SVA ≥ 100 mm this difference was absent (OR = 0.95; P = 0.96). It suggests that SPO has benefit only in cases with small or moderate sagittal imbalance. While this seems intuitive, this finding requires further confirmation due to the relatively small number of cases in the studied subgroups after the stratification. The same authors, previously cited, suggested that fixation to sacrum is associated with a higher risk of MC (OR = 3.7)[11]. The results of current study confirm this finding with important details: Fixation to sacrum with or without fixation to pelvis was associated with instrumentation failure (OR = 4.0, Table 9), in particular SL (OR = 2.5, Table 10). However, it simultaneously minimized risk of DSF as would be expected (OR = 0.08, Table 8). Inoue et al[16] (2015) showed that preoperative SVA > 95 mm is a risk factor of MC (HR = 2.6). Our results confirmed this finding with somewhat higher SVA threshold (SVA > 110 mm, OR = 4.5, Table 6). Combining of these findings by the Bayesian method suggested strong evidence (PO = 11.7) that severe preoperative anterior sagittal imbalance contributes to the risk of postoperative MC. Smoking was shown as a risk factor of MC (HR = 3.3)[16]. It was also confirmed by the present study, particularly for VF (OR = 5.7) and PJF (OR = 4.2), with a strong level of evidence by the Bayesian method (PO > 13.8). Smith et al[33] (2015) reported that postoperative SVA < 50 mm is a risk factor of PJF; however, adequate risk analysis and acceptable interpretation of this finding were not shown. The results of the current study have added details necessary for adequate interpretation of this finding. It was shown that the postoperative SVA < 50 mm can be associated with MC (SL), but specifically in patients with a preoperative SVA > 110 mm. This suggests significant correction, not SVA < 50 mm, is the more important factor to consider. Yagi et al[32] (2012) showed that progress of proximal junctional kyphosis is more significant in patients with osteoporosis. The results of present study confirmed the role of osteoporosis as a risk factor of PJF, specifically in cases after PSO and more than 5 levels fused. The obtained results regarding risk factors of RF are close to those previously published[27]. However, a few additional factors were revealed which are linked with severity of the preoperative sagittal imbalance and level of correction (Table 10). A relatively high incidence of SL appears contradictory to the experimental data, which showed that force around 1300N is necessary to cause pedicle screw failure. Supplemental hooks have not been shown to change this force[45]. Forces in the fusion construct are considerably less, but they act constantly during a long period of time which can cause permanent strain (micromotion). This strain may stimulate bone remodeling, decreasing contact surface between screws and the bone[46,47]. Finally, it may result in screw pullout (Figure 8). This process may take anywhere from one to several months.
In the current study we did not reveal a significant association between MC and such demographic characteristics as age, gender and BMI unlike some previous studies[8,35-37]. It may be explained by more severe preoperative sagittal imbalance in the cases that were included in the present study. This suggests the impact of factors other than demographic data prevailed in the studied case series. Devices and techniques for preoperative planning of correction were recently introduced at our institution[48]. These utilize patient specific rods precontoured by the manufacturer according to a preoperative surgical plan. The present study has shown that the use of this approach decreases the risk of the instrumentation failure (Table 9).
Analysis of the risk factors as presented above allows assumption that permanent mechanical stress in the spine and in the implanted devices, as a result of spinal correction, is the main risk of MC. The mechanical strength of bone and ligaments is less than that of the instrumentation; therefore MC of the spine occurred earlier than MC of instrumentation. Other factors such as surgical technique, type of instrumentation, and preoperative health status of patient may significantly modify the effect of this constant stress. This statement corresponds well with the previous experimental data suggesting that stiff instrumentation, which provides stability, simultaneously increases strain in the construct through a physiologic range of motion[49]. There are therefore two main sources of the postoperative mechanical stress. First is proportional to the preoperative spinal deformity, sagittal imbalance, abnormality of the spinopelvic alignment, and the level of correction; second is caused by the patient’s postoperative posture and motion. The first maybe increased by over-correction and the second may be worsened by insufficient correction. The combination of these 2 main effects causes somewhat contradictory results. An optimal balance between these two mechanical stresses is important to minimize the risk of the postoperative MC.
The results of the current and previous studies provide guidelines that may decrease the risk of postoperative MC. First, the absolute difference between postoperative and preoperative spinopelvic parameters should not exceed SVA > 75 mm, LL > 30°, TK > 25°, PI-LL > 30°, and PT > 9°. Second, postoperative anterior sagittal imbalance should not exceed 75 mm, and in patients with preoperative SVA > 110 mm, a postoperative SVA from 50 mm to 75 mm may be regarded as an acceptable. Third, in situ contouring of rods > 60° and repetitive contouring should be avoided. Fourth, the use of dominos and/or parallel connectors should be avoided or minimized. Fifth, the combination of pedicle screws with hooks may be appropriate in cases with preoperative SVA > 100 mm, having concomitant osteoporosis, and requiring significant correction with long posterior instrumented fusion (> 5 levels) and an osteotomy. Sixth, in cases with preoperative SVA < 100 mm, SPO is preferred to a PSO, if there is no specific indication for a PSO (such as ankylosing spondylitis) and adequate correction may be obtained. Finally, the use of preoperative planning with precontoured rods decreases the risk of instrumentation failure. The protective effect of this method may be enhanced by the application of optimal spinopelvic parameters, which provide criteria correction[50].
This study had several limitations, including the retrospective design with an inherent risk of selection bias and the incomplete/limited quality of the radiographic data, which may cause underestimation of significance for several of the studied risk factors. In particular, the role of sacral slope and pelvic incidence was not evaluated in the current study. The causes of the postoperative falls were not studied, and it is still unclear whether it was consequences of vestibular, vascular, mental or other diseases. The revealed risk factors, in spite of high statistical significance, had limited predictive capability, in particular, low positive predictive value. There may other risk factors that were not taken into consideration in the current study. However, in spite of these limitations, the combination of obtained results with the previously published findings confirms the consistency of the revealed effects. Therefore, the presented results should be viewed as a grounded, preliminary basis for further research with higher levels of evidence.
Incidence of MC after surgical correction of ADS is relatively high, and often requires additional surgical treatment. To diminish the risk of MC, the correction of sagittal imbalance and spinopelvic alignment should be appropriate, over- and insufficient correction should be avoided. Treatment strategy, surgical technique, and instrumentation should be improved for cases with severe anterior sagittal imbalance, spine compromised by previous surgical interventions, and, specifically, with concomitant osteoporosis.
It has been pointed out during last decades that mechanical complications (MC) after surgical correction of adult spine deformity (ASD) are most typical, and often require additional surgical treatment. However, these complications were not clearly defined. Their specific appearances, incidence, distribution by postoperative follow-ups, and risk factors were not studied well.
New knowledge concerning nature and causes of the MC would enable diminish their occurrence and improve postoperative clinical outcomes after surgical correction of ASD.
The main objectives of the study were identification of the most clinically relevant MC seen after surgical correction of ASD with corrective osteotomies, defining of their incidence, the most likelihood period of occurrence, association with additional surgeries; revealing of risk factors and assessment their predictive value. Achievement of these purposes would have enabled formulation of grounded recommendation to diminish risk of such complications and contribute to defining directions for further research in this field.
The retrospective clinical study was performed. Medical records, operation protocols, and radiographic images were studied in patients who underwent surgical correction of adult spine deformity with osteotomy. Preoperative, perioperative, and postoperative data were collected for 2 and more years of follow-up. Postoperative mechanical failures of spine and implanted instrumentation were studied in detail including: their features, latent periods, incidence, required additional treatment, and different risk factors such as: Demographic, preoperative and postoperative spinopelvic alignment, level of correction, spinal instrumentation, features of surgical intervention, etc.
It was shown that around half of patients experienced MC during two postoperative years; majority of these cases required additional surgery. MC of spine occurred earlier and more often required revision than breakage of the instrumentation. The main risk factors included severe preoperative sagittal imbalance, inadequate correction of the spinopelvic alignment, preoperative comorbidities (osteoporosis, smoking), postoperative events (falls), and features of the spinal instrumentation. There was developed method that enables recognition of patients with high risk of postoperative MC.
The performed study is first that performed a clear classification of the clinically relevant MC after surgical correction of ASD with osteotomy. In particular, there were specified those complications that are linked with failure of spine, breakage of the instrumentation; and disassociation between different elements of the spinal fusion construct. First time, impact of more than 50 potential risk factors of the MC and their combinations was assessed. There were revealed risk factors and their combinations that had statistically significant association with one or a few MC. The predictive value of each of these risk factors for each type of MC was evaluated. The obtained results allowed development of a new method to recognize patients with high risk of postoperative MC; and provide newel grounded recommendations to diminish risk of such complications. Implication for clinical practice: implementation of these methods can contribute to improvement of treatment outcomes after surgical correction of ASD with osteotomy, and diminish treatment expenses.
The obtained results and recommendations require further confirmation by studies with higher level of evidence such as prospective cohort and randomized clinical trials. The predictive capability of the risk factors revealed in the current study showed underestimation of risk of MC after surgical correction of ASD. It suggests that other currently unknown risk factors likely also exist. Therefore, further researches are needed in this field to reveal these factors.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Pani SP, Petretta M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 2. | Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 130] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Gill JB, Levin A, Burd T, Longley M. Corrective osteotomies in spine surgery. J Bone Joint Surg Am. 2008;90:2509-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Bridwell KH. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. vertebral column resection for spinal deformity. Spine (Phila Pa 1976). 2006;31:S171-S178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Enercan M, Ozturk C, Kahraman S, Sarıer M, Hamzaoglu A, Alanay A. Osteotomies/spinal column resections in adult deformity. Eur Spine J. 2013;22 Suppl 2:S254-S264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Buchowski JM, Bridwell KH, Lenke LG, Kuhns CA, Lehman RA Jr, Kim YJ, Stewart D, Baldus C. Neurologic complications of lumbar pedicle subtraction osteotomy: a 10-year assessment. Spine (Phila Pa 1976). 2007;32:2245-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Cho KJ, Bridwell KH, Lenke LG, Berra A, Baldus C. Comparison of Smith-Petersen versus pedicle subtraction osteotomy for the correction of fixed sagittal imbalance. Spine (Phila Pa 1976). 2005;30:2030-2037; discussion 2038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Kim YJ, Bridwell KH, Lenke LG, Cheh G, Baldus C. Results of lumbar pedicle subtraction osteotomies for fixed sagittal imbalance: a minimum 5-year follow-up study. Spine (Phila Pa 1976). 2007;32:2189-2197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | Yang BP, Ondra SL, Chen LA, Jung HS, Koski TR, Salehi SA. Clinical and radiographic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance. J Neurosurg Spine. 2006;5:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Ikenaga M, Shikata J, Takemoto M, Tanaka C. Clinical outcomes and complications after pedicle subtraction osteotomy for correction of thoracolumbar kyphosis. J Neurosurg Spine. 2007;6:330-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Charosky S, Guigui P, Blamoutier A, Roussouly P, Chopin D; Study Group on Scoliosis. Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients. Spine (Phila Pa 1976). 2012;37:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Cho SK, Bridwell KH, Lenke LG, Yi JS, Pahys JM, Zebala LP, Kang MM, Cho W, Baldus CR. Major complications in revision adult deformity surgery: risk factors and clinical outcomes with 2- to 7-year follow-up. Spine (Phila Pa 1976). 2012;37:489-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Chang KW, Cheng CW, Chen HC, Chang KI, Chen TC. Closing-opening wedge osteotomy for the treatment of sagittal imbalance. Spine (Phila Pa 1976). 2008;33:1470-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Smith JS, Shaffrey CI, Ames CP, Demakakos J, Fu KM, Keshavarzi S, Li CM, Deviren V, Schwab FJ, Lafage V. Assessment of symptomatic rod fracture after posterior instrumented fusion for adult spinal deformity. Neurosurgery. 2012;71:862-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Smith JS, Klineberg E, Lafage V, Shaffrey CI, Schwab F, Lafage R, Hostin R, Mundis GM Jr, Errico TJ, Kim HJ, Protopsaltis TS, Hamilton DK, Scheer JK, Soroceanu A, Kelly MP, Line B, Gupta M, Deviren V, Hart R, Burton DC, Bess S, Ames CP; International Spine Study Group. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 16. | Inoue S, Khashan M, Fujimori T, Berven SH. Analysis of mechanical failure associated with reoperation in spinal fusion to the sacrum in adult spinal deformity. J Orthop Sci. 2015;20:609-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976). 2007;32:2238-2244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | Emami A, Deviren V, Berven S, Smith JA, Hu SS, Bradford DS. Outcome and complications of long fusions to the sacrum in adult spine deformity: luque-galveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976). 2002;27:776-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Cho KJ, Suk SI, Park SR, Kim JH, Kim SS, Choi WK, Lee KY, Lee SR. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine (Phila Pa 1976). 2007;32:2232-2237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 20. | Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O’Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976). 2010;35:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Yadla S, Maltenfort MG, Ratliff JK, Harrop JS. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus. 2010;28:E3. [PubMed] [Cited in This Article: ] |
| 22. | Blamoutier A, Guigui P, Charosky S, Roussouly P, Chopin D; Groupe d’Étude de la Scoliose (GES). Surgery of lumbar and thoracolumbar scolioses in adults over 50. Morbidity and survival in a multicenter retrospective cohort of 180 patients with a mean follow-up of 4.5 years. Orthop Traumatol Surg Res. 2012;98:528-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Auerbach JD, Lenke LG, Bridwell KH, Sehn JK, Milby AH, Bumpass D, Crawford CH 3rd, O Shaughnessy BA, Buchowski JM, Chang MS, Zebala LP, Sides BA. Major complications and comparison between 3-column osteotomy techniques in 105 consecutive spinal deformity procedures. Spine (Phila Pa 1976). 2012;37:1198-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 24. | Kim YJ, Bridwell KH, Lenke LG, Cho KJ, Edwards CC 2nd, Rinella AS. Pseudarthrosis in adult spinal deformity following multisegmental instrumentation and arthrodesis. J Bone Joint Surg Am. 2006;88:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Dickson DD, Lenke LG, Bridwell KH, Koester LA. Risk factors for and assessment of symptomatic pseudarthrosis after lumbar pedicle subtraction osteotomy in adult spinal deformity. Spine (Phila Pa 1976). 2014;39:1190-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Le Huec JC, Cogniet A, Demezon H, Rigal J, Saddiki R, Aunoble S. Insufficient restoration of lumbar lordosis and FBI index following pedicle subtraction osteotomy is an indicator of likely mechanical complication. Eur Spine J. 2015;24 Suppl 1:S112-S120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Barton C, Noshchenko A, Patel V, Cain C, Kleck C, Burger E. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: a retrospective case-series. Scoliosis. 2015;10:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Annis P, Lawrence BD, Spiker WR, Zhang Y, Chen W, Daubs MD, Brodke DS. Predictive factors for acute proximal junctional failure after adult deformity surgery with upper instrumented vertebrae in the thoracolumbar spine. Evid Based Spine Care J. 2014;5:160-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Lonner BS, Newton P, Betz R, Scharf C, O’Brien M, Sponseller P, Lenke L, Crawford A, Lowe T, Letko L. Operative management of Scheuermann’s kyphosis in 78 patients: radiographic outcomes, complications, and technique. Spine (Phila Pa 1976). 2007;32:2644-2652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Kim YJ, Lenke LG, Bridwell KH, Kim J, Cho SK, Cheh G, Yoon J. Proximal junctional kyphosis in adolescent idiopathic scoliosis after 3 different types of posterior segmental spinal instrumentation and fusions: incidence and risk factor analysis of 410 cases. Spine (Phila Pa 1976). 2007;32:2731-2738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Maruo K, Ha Y, Inoue S, Samuel S, Okada E, Hu SS, Deviren V, Burch S, William S, Ames CP. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine (Phila Pa 1976). 2013;38:E1469-E1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 240] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 32. | Yagi M, King AB, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine (Phila Pa 1976). 2012;37:1479-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 33. | Smith MW, Annis P, Lawrence BD, Daubs MD, Brodke DS. Acute proximal junctional failure in patients with preoperative sagittal imbalance. Spine J. 2015;15:2142-2148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Wang J, Zhao Y, Shen B, Wang C, Li M. Risk factor analysis of proximal junctional kyphosis after posterior fusion in patients with idiopathic scoliosis. Injury. 2010;41:415-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | O’Leary PT, Bridwell KH, Lenke LG, Good CR, Pichelmann MA, Buchowski JM, Kim YJ, Flynn J. Risk factors and outcomes for catastrophic failures at the top of long pedicle screw constructs: a matched cohort analysis performed at a single center. Spine (Phila Pa 1976). 2009;34:2134-2139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Bridwell KH, Lenke LG, Cho SK, Pahys JM, Zebala LP, Dorward IG, Cho W, Baldus C, Hill BW, Kang MM. Proximal junctional kyphosis in primary adult deformity surgery: evaluation of 20 degrees as a critical angle. Neurosurgery. 2013;72:899-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 37. | Kim HJ, Bridwell KH, Lenke LG, Park MS, Song KS, Piyaskulkaew C, Chuntarapas T. Patients with proximal junctional kyphosis requiring revision surgery have higher postoperative lumbar lordosis and larger sagittal balance corrections. Spine (Phila Pa 1976). 2014;39:E576-E580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 38. | Hennekens CH, Buring JE, Mayrent SL. Epidemiology in medicine. 1st ed. Boston: Little, Brown 1987; 383. [Cited in This Article: ] |
| 39. | Lafage V, Schwab F, Patel A, Hawkinson N, Farcy JP. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976). 2009;34:E599-E606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 796] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 40. | Schwab F, Patel A, Ungar B, Farcy JP, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976). 2010;35:2224-2231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 733] [Cited by in F6Publishing: 785] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 41. | Glantz SA. Primer of biostatistics. New York: McGraw-Hill Medical Pub 2005; 520. [Cited in This Article: ] |
| 42. | Goodman SN. Toward evidence-based medical statistics. 2: The Bayes factor. Ann Intern Med. 1999;130:1005-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 606] [Cited by in F6Publishing: 527] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 43. | Goodman SN. Introduction to Bayesian methods I: measuring the strength of evidence. Clin Trials. 2005;2:282-290; discussion 301-304, 364-378. [PubMed] [Cited in This Article: ] |
| 44. | Lau D, Clark AJ, Scheer JK, Daubs MD, Coe JD, Paonessa KJ, LaGrone MO, Kasten MD, Amaral RA, Trobisch PD. Proximal junctional kyphosis and failure after spinal deformity surgery: a systematic review of the literature as a background to classification development. Spine (Phila Pa 1976). 2014;39:2093-2102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 45. | Tai CL, Chen LH, Lee DM, Liu MY, Lai PL. Biomechanical comparison of different combinations of hook and screw in one spine motion unit--an experiment in porcine model. BMC Musculoskelet Disord. 2014;15:197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 46. | Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;S132-S147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 434] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 47. | Frost HM. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004;74:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 33] [Reference Citation Analysis (0)] |
| 48. | Barton C, Noshchenko A, Patel V, Kleck C, Burger E. Early Experience and Initial Outcomes With Patient-Specific Spine Rods for Adult Spinal Deformity. Orthopedics. 2016;39:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Kleck CJ, Illing D, Lindley EM, Noshchenko A, Patel VV, Barton C, Baldini T, Cain CM, Burger EL. Strain in Posterior Instrumentation Resulted by Different Combinations of Posterior and Anterior Devices for Long Spine Fusion Constructs. Spine Deform. 2017;5:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Noshchenko A, Hoffecker L, Cain CMJ, Patel VV, Burger EL. Spinopelvic Parameters in Asymptomatic Subjects Without Spine Disease and Deformity: A Systematic Review With Meta-Analysis. Clin Spine Surg. 2017;30:392-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |