Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9520
Peer-review started: February 24, 2021
First decision: April 20, 2021
Revised: May 4, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 6, 2021
Glycated albumin (GA), the non-enzymatic glycation product of albumin in plasma, became a glycemic marker in the beginning of the 21st century. The assay is not affected by hemoglobin levels and reflects the glycemic status over a shorter period as compared to HbA1c measurements. Thus, GA may contributes as an intermediate glucose index in the current diabetes mellitus (DM) diagnostic system.
To search and summarize the available data on glycated albumin measurements required for the diagnosis of diabetes mellitus.
Databases, including PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL), among others, were systematically searched. The Quality Assessment of Diagnostic Accuracy Studies-2 tool was applied for the assessment of quality, and the bivariate model was used to pool the sensitivity and specificity. The hierarchical summary receiver operator characteristic curves (HSROC) model was utilized to estimate the summary receiver operating characteristics curve (SROC). Sensitivity analysis was performed to investigate the association of the study design and patient characteristics with the test accuracy and meta-regression to find the source of heterogeneity.
Three studies regarding gestational diabetes mellitus (GDM) and a meta-analysis of 16 non-GDM studies, comprising a total sample size of 12876, were included in the work. Results reveal that the average cut-off values of GA reported for the diagnosis of GDM diagnosis was much lower than those for non-GDM. For non-GDM cases, diagnosing DM with a circulating GA cut-off of 14.0% had a sensitivity of 0.766 (95%CI: 0.539, 0.901), specificity of 0.687 (95%CI: 0.364, 0.894), and area under the curve of 0.80 (95%CI: 0.76, 0.83) for the SROC. The estimated SROC at different GA cut-off values for non-GDM exhibited that the average location parameter lambda of 16 non-GDM studies was 2.354 (95%CI: 2.002, 2.707), and the scale parameter beta was -0.163 (95%CI: -0.614, 0.288). These non-GDM studies with various thresholds had substantial heterogeneity, which may be attributed to the type of DM, age, and body mass index as possible sources.
Glycated albumin in non-DM exhibits a moderate diagnostic accuracy. Further research on the diagnostic accuracy of GA for GDM and combinational measure
Core Tip: This is the first systematic review and meta-analysis investigating the utility of glycated albumin (GA) for the diagnosis of diabetes mellitus (DM). Three studies regarding gestational diabetes mellitus (GDM) were included in the systematic review, and another 16 studies on non-GDM were included in the meta-analysis. This study found that the average cut-off values of GA reported for GDM diagnosis were much lower than those for non-GDM. The GA cut-off value of 14.0% exhibited a moderate diagnostic accuracy in non-GDM. GA, as the sole DM diagnostic test, should be interpreted with caution to assure the correct classification of diabetic individuals.
- Citation: Xiong JY, Wang JM, Zhao XL, Yang C, Jiang XS, Chen YM, Chen CQ, Li ZY. Glycated albumin as a biomarker for diagnosis of diabetes mellitus: A systematic review and meta-analysis. World J Clin Cases 2021; 9(31): 9520-9534
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9520.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9520
Diabetes mellitus (DM) is considered as a group of metabolic disorders characterized by hyperglycemia due to impaired insulin secretion or resistance to peripheral actions of insulin, or both[1]. The number of DM cases was 463 million in 2019 and is estimated to increase to 578 million by 2030 and to 700 million by 2045. Considering its projected increasing rates and detrimental side effects, DM has become a concerning epidemic worldwide[2]. Diabetes can lead to further acute complications, including diabetic ketoacidosis, non-ketotic hyperosmolar coma, and other progressive long-term complications, such as cardiovascular disease, chronic kidney failure, nerve damage, foot ulcers, retinopathy, and susceptibility to pathognomonic infection[3]. DM also causes considerable morbidity and premature mortality mainly due to the associated cardiovascular complications that worsen a patient’s condition[2].
Several approaches could reduce the risk of cardiovascular morbidity and mortality[4] associated with type 2 diabetes, which include early detection, periodic screening, and diagnosis of DM in the early stage of the disease. Similarly, multiple assessments to diagnose GDM during pregnancy are made especially between 24 and 28 gestational weeks to avoid severe maternal and fetal adverse events. Poor pregnancy outcomes have been reported despite the appropriate treatment, thus warranting early screening for GDM in these high-risk populations[5].
Glucose levels are greatly affected by diet, physical activity, mental state, illness, and medications. Universal methods to diagnose DM include fasting plasma glucose level (FPG), 2-h plasma glucose (2hPG), and random blood glucose (RBG), all of which reflect blood glucose at the time of blood collection. Amongst these short-term indices of glycaemia, 2-hour glucose levels substantially show more variability as compared with FPG[6]. To improve the simple diagnosis of DM, researchers have switched focus to diagnoses based on levels of hemoglobin A1c (HbA1c) and other indices, which further reflect the glycemic control over a long period of time. Over the past decade, several in-depth studies on HbA1c show that glycated proteins play key role in the diagnosis, prognosis, and assessment of diabetic severity. According to the ADA’s 2020 guidelines for the diagnosis of DM, 1/3 more undiagnosed diabetes cases are diagnosed using the HbA1c cut-point of ≥ 6.5% as compared to using glucose criteria alone[7].
The concordance between HbA1C and glucose-based tests is not satisfied[8]. Moreover, HbA1c is not a suitable method to diagnose DM when patients present with increased red blood cell turnover, which occurs during pregnancy (especially in the second and third trimesters), recent blood loss or transfusion, hemodialysis, or erythropoietin therapy[8]. Several studies have focused on fructosamine (FA)[9] and glycated albumin (GA)[10] assessment to reflect the intermediate-term glycemic control in patients with diabetes[11], which could be practiced when HbA1c is unavailable or its interpretation is problematic[12]. However, FA, an identifying biomarker for all glycated proteins, is strongly influenced by disorders of proteins, uric acid, urea, and other low-molecular-weight substances[13,14].
Glycated albumin is the non-enzymatic glycation product of albumin in the plasma and, thus, became a new glycemic marker in the beginning of the 21st century for the diagnosis of DM. While HbA1c is affected by the 12-wk lifespan of hemoglobin, the plasma concentration of GA is influenced by the duration of albumin, reflecting the average blood glucose 2-4 wk before measurements[15]. When blood glucose concentrations increase, approximately 4.5 times more GA is produced than HbA1c[16]; therefore, the concentration of GA increases faster than that of HbA1c. In previous works, GA was measured by chromatography, thiobarbituric acid calorimetry[17], immunoassays[18], electrophoretic techniques[19], label-free impedimetricimmuno sensor[20], and enzymatic methods[21]; however, most of these methods are laborious and unavailable in routine clinical laboratories. The most recently applied methods for the determination of GA include high-performance liquid chromatography (HPLC)[22], immunoassays, and enzymatic methods. For instance, Kohzuma et al[23] developed an enzymatic assessment of GA (LucicaVR GA-L, Asahi Kasei Pharma, Japan) in 2011, which has been widely applied for GA estimation in Japan and other Asian countries. Another enzymatic method, named quantILab Glycated Albumin assay[24], has proven to be suitable for clinical use and is primarily available in Europe. Considering the absence of an international standard for GA detection, this study investigated the different cut-off values for the diagnoses of DM with no limitations.
GA has been verified for various aspects in the management of DM in clinical chemistry. For instance, monitoring glucose excursions specifically reflects postprandial[25] glucose levels and helps to guide the treatment strategies for DM[26]. GA has been strongly correlated with the development of diabetes complications, especially retinopathy and nephropathy[27], and has also been proposed as a marker for the assessment of atherosclerosis risk and coronary artery diseases[10]. Lan et al[28] found that the ratio of serum glycated albumin to glycated hemoglobin (GA/HbA1c) potentially differentiates occult DM from fulminant type 1 diabetes. According to the diagnosis aspect, researches on the diagnostic value of GA in DM have mostly been conducted in various regions of Asia. Zhou et al[29] reported the reference value of GA to range 10.23-14.79% for the Chinese healthy population, which statistically differs according to three age groups and gender. Matsha et al[30] found that the 2.5 and 97.5 percentiles of GA were 10.7% and 15.1%, respectively, in South Africa, and the reference limits of GA vary with body mass index (BMI). Bellia et al[31] evaluated 334 Caucasians and determined that the GA presented a sensitivity and specificity of 72.2% and 71.8%, respectively, using the quantILab glycated albumin assay. Hsu et al[32] described a cutoff point of 14.9% GA for DM in 2192 adults in Taiwan (sensitivity: 66.4%; specificity: 88.3%) using the Lucica GA-L kit.
Since GA assays do not require fasting blood samples[33] and are relatively cheap[34], they can be performed on the same tube used for routine biochemical tests and can reduce the frequency of invasive procedures as compared to OGTT. Unlike HbA1c measurements, GA is not affected by hemoglobin levels and reflects the glycemic status over a shorter period of time. Thus, GA can contribute as an intermediate glucose index in the current DM diagnostic system, which has been missing.
In this study, we performed a systematic review and meta-analysis aiming to summarize and assess the diagnostic data to evaluate the suitability of GA in the diagnosis of DM.
This study was performed and reported according to the guidance of Preferred Reporting Items for Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA)[35], with PROSPERO registration number: CRD42020164315. The RevMan5.3 software and STATA 14.0 software were used for the assessment of data and statistical analyses. Two investigators independently evaluated the paper selection, extracted the data from published paper, and assessed the quality of the included studies. In case of difference of opinion, discussions were conducted among the entire study team to make the final decisions.
A comprehensive literature search was performed to assess the accuracy of GA for the diagnosis of DM using the following databases: PubMed, Medline, EMBASE, Cochrane CENTRAL, Web of Science, Chinese National Knowledge Infrastructure (CNKI), VIP, Chinese Medical Association (CMA), and Digital Periodicals of Wanfang. The reference list of selected papers was hand-searched; Clinicaltrails.gov was searched for unpublished research and Opengrey EU for the gray literature. Two extensive searches were performed, the first search on November 30, 2019 and the second on November 30, 2020. The search terms included “glycosylated serum albumin,” “diabetes mellitus,” and “diagnosis,” and the thesauruses of these Mesh/Emtree terms combined with Boolean rules, which are specified in different databases, to ensure that all the relevant literature was included. For the PubMed database, the following keyword combinations were used: (type 2 diabetes mellitus OR T2DM OR type 1 diabetes mellitus OR T1DM OR gestational diabetes mellitus OR GDM)[Title/Abstract] AND (Glycosylated serum albumin OR glycated albumin)[Title/Abstract] AND (Diagnosis OR screening OR Diagnostic accuracy OR Sensitivity OR Specificity[Title/Abstract]) (Supplementary material).
The retrieved articles were dentified and initially screened by their titles and abstracts to include the relevant works and exclude the irrelevant studies. Full-text articles were then searched to select the possible inclusion pieces of literature, according to the inclusion criteria as mentioned below.
The criteria for the searched studies considered: (1) Population inclusion, specifi
The population exclusion criteria included individuals with leukocythemia, therioma, thyroid diseases, abnormal liver and kidney function, acute infection, and severe cardiovascular diseases. Case reports, review studies, and case-control studies, which compared the test results in ‘case’ with the severe disease to those in healthy ‘controls,’ were excluded from this study.
Articles published in English or Chinese were searched and were excluded if they did not provide sufficient data, such as TP (true positive), FP (false positive), TN (true negative), and FN (false negative), levels of sensitivity, specificity, and positive and negative predictive values to construct the 2 × 2 contingency table. We collected the following information from each study: General information (author, publication year, geographic region, mean age, and BMI of the population involved), sample size, study design, type of assay to measure GA, the cut-off value of GA for diagnosis of DM, and the test and diagnostic data mentioned above.
Quality assessment was conducted by applying Quality Assessment of Diagnostic Accuracy Studies[36] which contains four domains, i.e., patient selection, index test, reference standard, and flow and timing domains. If a study was judged as “high” or “unclear” in one or more domains, then it was deemed “at risk of bias” or as having “concerns regarding applicability.” Tabular and graphic displays were generated with RevMan5.3 software to summarize the QUADAS-2 assessments.
The diagnostic accuracy was estimated by a summary point using a bivariate model for studies with a common threshold and by an SROC curve using HSROC model to describe studies with many thresholds. Pooled sensitivity, specificity, area under the curve (AUC) with 95%CI, and SROC were analyzed with STATA 14.0 software. RevMan5.3 software was used to extract or calculate TP, FP, TN, and FN values.
Since heterogeneity was presumed to exist in diagnostic reviews, a random effects model was used as the default method, unless very few studies, estimating between-study variability or analysis, demonstrated that a fixed effects model was appropriate. The pre-planned subgroups were divided according to the type of DM, BMI, age, and method by which GA was measured. The magnitude of the heterogeneity was depicted by plotting the prediction region in ROC space. Heterogeneity is high when the 95% prediction region is much larger than the 95% confidence region, and vice versa. The potential source of heterogeneity was investigated by meta-regression through STATA 14.0 or SAS statistical software, which uses covariates like cut-off values, population region, age, BMI, sex, study-type, sample size, standard test, and the assay used to measure GA. When certain studies were excluded, sensitivity analysis was performed to analyze the stability of the diagnostic statistical value of GA for diagnosing DM, accounting for significant heterogeneity and meta-regression or risk of bias. Subgroups were developed as the individual heterogeneity resources were identified by meta-regression when possible. We further assessed the risk of publication bias using Deek’s Funnel plot.
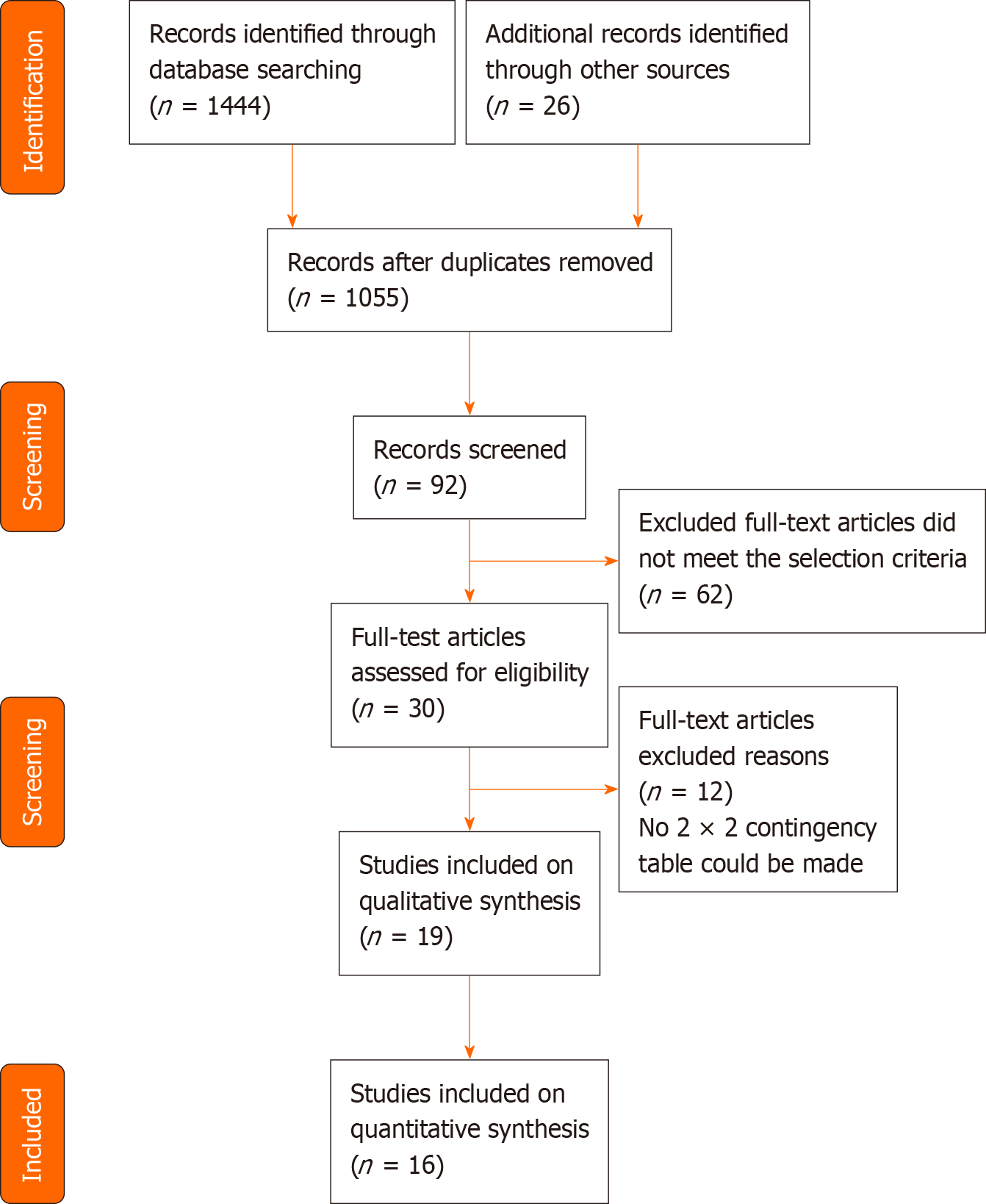
The selection process of published studies from the available literature is presented in Figure 1. A total of electronically searched 1444 articles in PubMed (n = 134), EMBASE (n = 239), Cochrane Library (n = 145), Web of Science (n = 194), MEDLINE (n = 192), Scopus (n = 219), Chinese National Knowledge Infrastructure (CNKI) (n = 36), VIP (n = 143), and Chinese Medical Association (CMA) Digital Periodicals of Wanfang (n = 142) were enrolled in this study. Records (n = 26) were obtained from Clinicaltrails.gov and WHO ICTRP. No other studies were extracted from the reference review of the selected studies or the hand-search of other sources.
After eliminating the duplicated papers (n = 415) and cross-checking the title and abstract for inappropriate articles (n = 963), 92 full-text articles were scrutinized for further evaluation. Finally, out of the 30 articles that matched the selection criteria, we excluded additional studies that did not report diagnostic test accuracy data, did not mention the gold standard reference, or referred to the same study population as presented in another study. The remaining 19 studies included 3 on GDM in the systematic review[37-39] and 16 on non-GDM in the meta-analysis. Of the latter 16 studies[33,40-54], 3 articles reported special types of DM, including cystic fibrosis-related diabetes (CFRD) or post-transplant diabetes mellitus (PTDM). Except for a retrospective cross-sectional study by Wang et al[41], the remaining studies were prospective cross-sectional or cohort studies. The average age of participants was 47.7-59 years in the 13 non-GDM studies, 14.2 years in CFRD, 36-46.1 years in PTDM, and mostly 24-28 gestational weeks in GDM subjects. Ten studies measured GA with Lucica GA-L[23], which was developed in Japan and has been verified for its excellent performance of accuracy. In two studies, GA was measured with GlycoGap[55], and one study measured GA with the quantILab Glycated Albumin assay[24]. No specified enzymatic method or HPLC was conducted respectively in the other two studies. Detailed information on the selected studies is shown in Table 1.
| Ref. | Year | Nation | Men, n (%) | Age (yr) | BMI (kg/m2)/BMI z-score | Detection methods | Cut-off | n | TP (a) | FP (b) | FN (c) | TN (d) |
| Chume et al[42] | 2019 | Brazil | 40.5 | 53.41 | 28.91 | GlycoGap, Poway, CA | 14.8% | 242 | 50 | 57 | 27 | 108 |
| Furusyo et al[52] | 2011 | Japan | 29.8 | 49.9 | 22.2 | Lucica GA-L, Japan | 15.5% | 1575 | 60 | 251 | 12 | 1252 |
| He et al[47] | 2017 | China | 55.2 | 55.0 | 24.9 | Lucica GA-L, Japan | 17.1% | 1287 | 629 | 12 | 363 | 283 |
| Ikezaki et al[48] | 2015 | Japan | 32.0 | 59.0 | 23.1 | Lucica GA-L, Japan | 15.2% | 908 | 36 | 56 | 22 | 91 |
| Lang et al[37] | 2018 | China | 0 | 20-30 wk2 | Not given | Enzymatic method,Japan | 13.5% | 276 | 104 | 42 | 35 | 95 |
| Liu et al[38] | 2020 | China | 0 | 24-28 wk | 25.1 | LST 008 | 14.2% | 261 | 66 | 76 | 20 | 99 |
| Ma et al[53] | 2010 | China | 45.4 | 53.1 | 24.2 | Lucica GA-L, Japan | 17.1% | 1971 | 580 | 281 | 175 | 935 |
| Pimentel et al[40] | 2020 | Brazil | 53.0 | 46.1 | 26.0 | GlycoGap, Diazyme Laboratories | 16.5% | 134 | 17 | 16 | 16 | 85 |
| Shima et al[54] | 1989 | Japan | 83.3 | 47.7 | Not given | HPLC | 21.6% | 302 | 40 | 21 | 9 | 232 |
| Su et al[46] | 2018 | China | 46.7 | 50.5 | 24.7 | Lucica GA-L, Japan | 16.3% | 691 | 226 | 59 | 109 | 297 |
| Su et al[45] | 2019 | China | 46.2 | 50.4 | 24.7 | Lucica GA-L, Japan | 17.1% | 701 | 190 | 40 | 160 | 311 |
| Tommerdah et al[44] | 2019 | United States | 41.0 | 14.2 | −0.023 | Lucica GA-L, Japan | 14.0% | 58 | 2 | 5 | 7 | 44 |
| Wang et al[41] | 2020 | China | 69.5 | 36.0 | 20.6 | Not given | 14.6% | 210 | 12 | 24 | 9 | 165 |
| Wu et al[33] | 2016 | Taiwan | 40.0 | 50.4 | 24.3 | Lucica GA-L, Japan | 15.0% | 1559 | 98 | 208 | 34 | 1219 |
| Zemlin et al[43] | 2019 | South Africa | 25.8 | 47.8 | 28.7 | Werfen, Italy | 14.9% | 1294 | 62 | 77 | 32 | 1123 |
| Zhang et al[50] | 2014 | China | 49.5 | 50.2 | 25.0 | Lucica GA-L, Japan | 16.6% | 392 | 94 | 33 | 37 | 228 |
| Zheng[51] | 2012 | China | 52.7 | 51.9 | Not given | Enzymatic method, Japan | 17.5% | 509 | 223 | 16 | 95 | 175 |
| Zhuang et al[39] | 2019 | China | 0 | 24-28 wk | 20.6 | Colorimetric method | 11.5% | 357 | 145 | 94 | 41 | 77 |
| Hwang et al[49] | 2014 | South Korea | 58.5 | 52.5 | 24.8 | Lucica GA-L, Japan | 14.3% | 852 | 210 | 63 | 106 | 473 |
Most of the studies included the diagnostic criteria for DM specified by the World Health Organization (WHO, 1999) guidelines, i.e., FPG ≥ 7.0 mmol/L or 2-h post-load PG ≥ 11.1 mmol/L or RBG ≥ 11.1 mmol/L. The other studies employed ADA criteria, which consider HbA1c ≥ 6.5% as the criterion for DM. The diagnosis of GDM in all the three studies was reported for any one of the following plasma glucose values: FPG ≥ 5.1 mmol/L, 1-h post-load PG ≥ 10.0 mmol/L, and 2-h post-load PG ≥ 8.5 mmol/L. Considering our objectives of this meta-analysis, prediabetes impaired glucose regulation was listed as non-diabetes. The data were then used to prepare the statistical 2 × 2 contingency table.
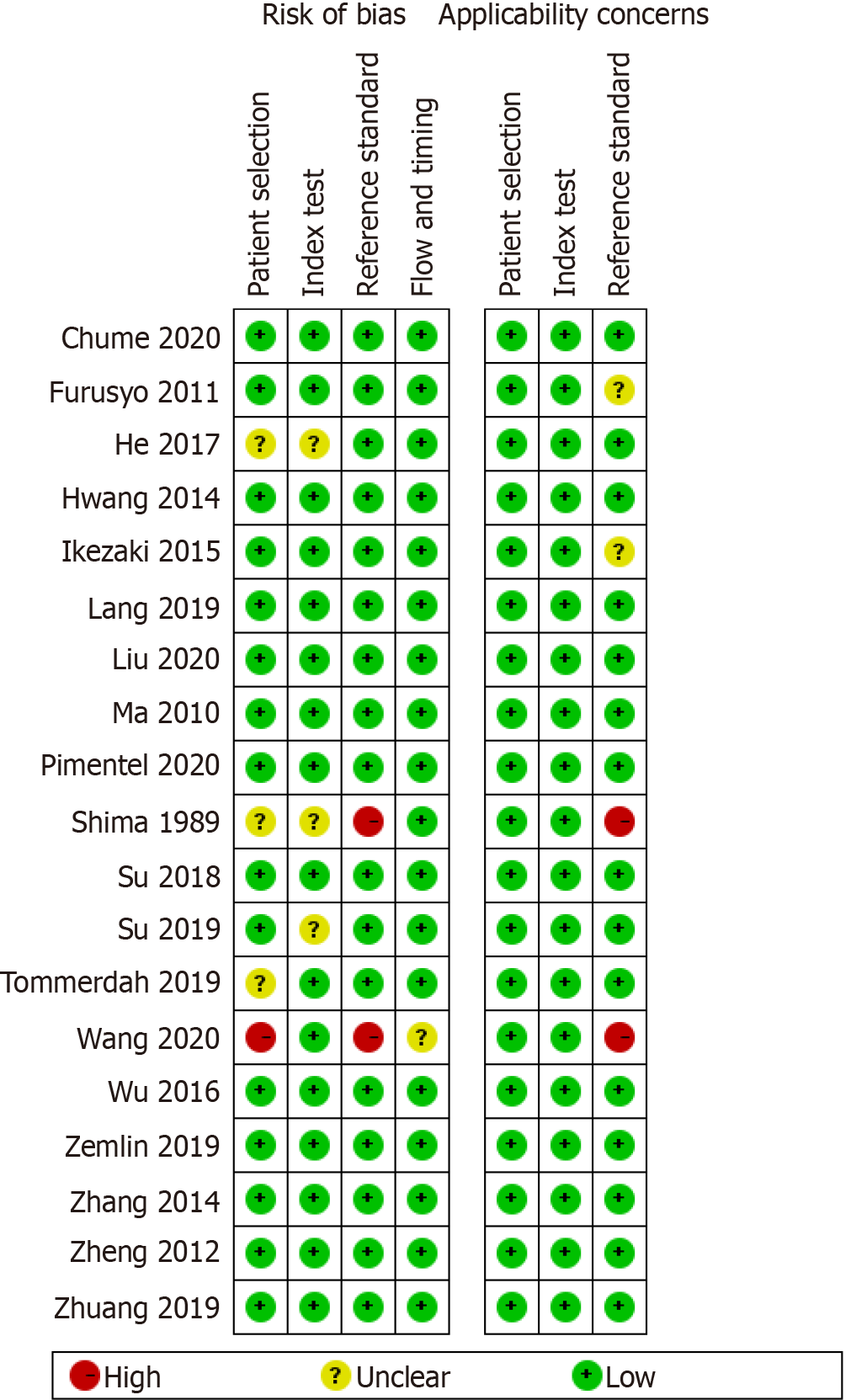
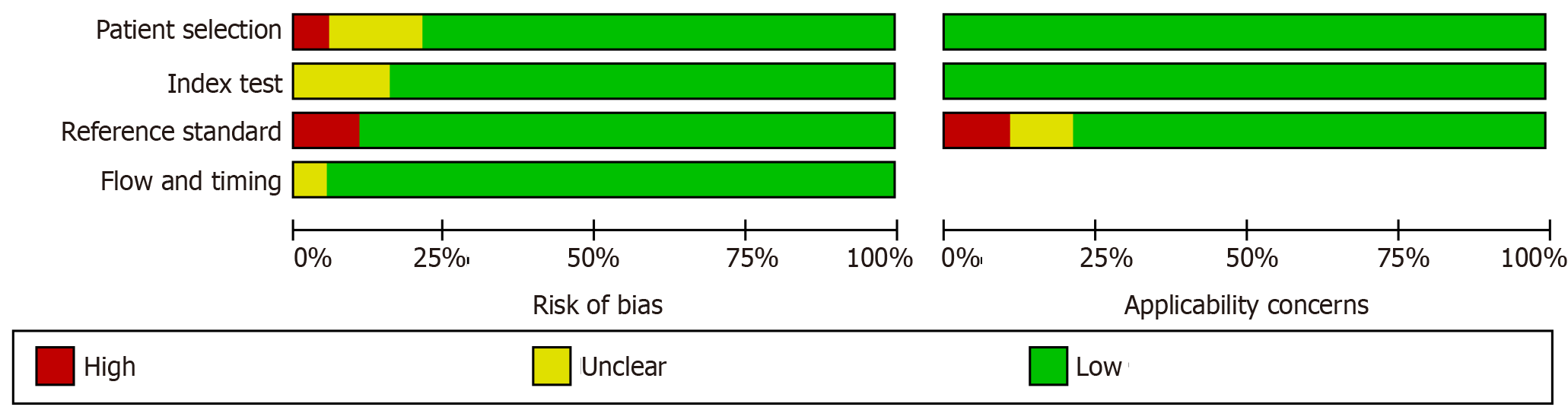
The methodological quality of the selected studies was assessed using the QUADAS-2 tool (Figures 2 and 3). For patient selection, one study (Wang et al[41]) was ranked as “high risk” for not mentioning the random patient selection, three[44,47,54] were ranked as “unclear risk,” and others were identified as having a low bias risk. Most of the studies were identified for having a low risk of bias and high quality, apart from two studies[41,54] that showed a high risk of reference standard because of inconsistency with the majority of the studies. One study (Wang et al[41]) was considered as having an unclear risk for the item flow and timing due to exclusion of some positive and negative subjects.
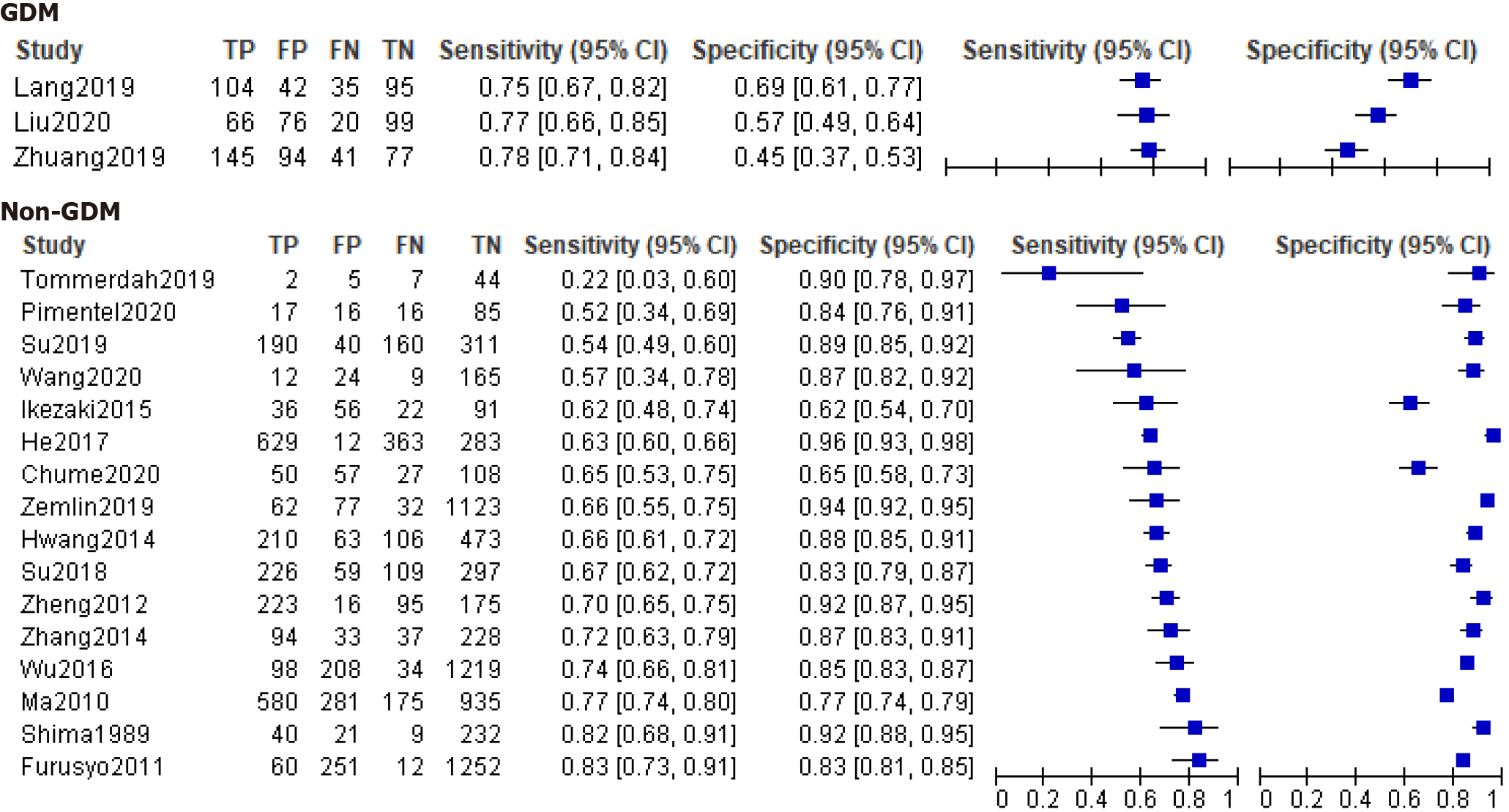
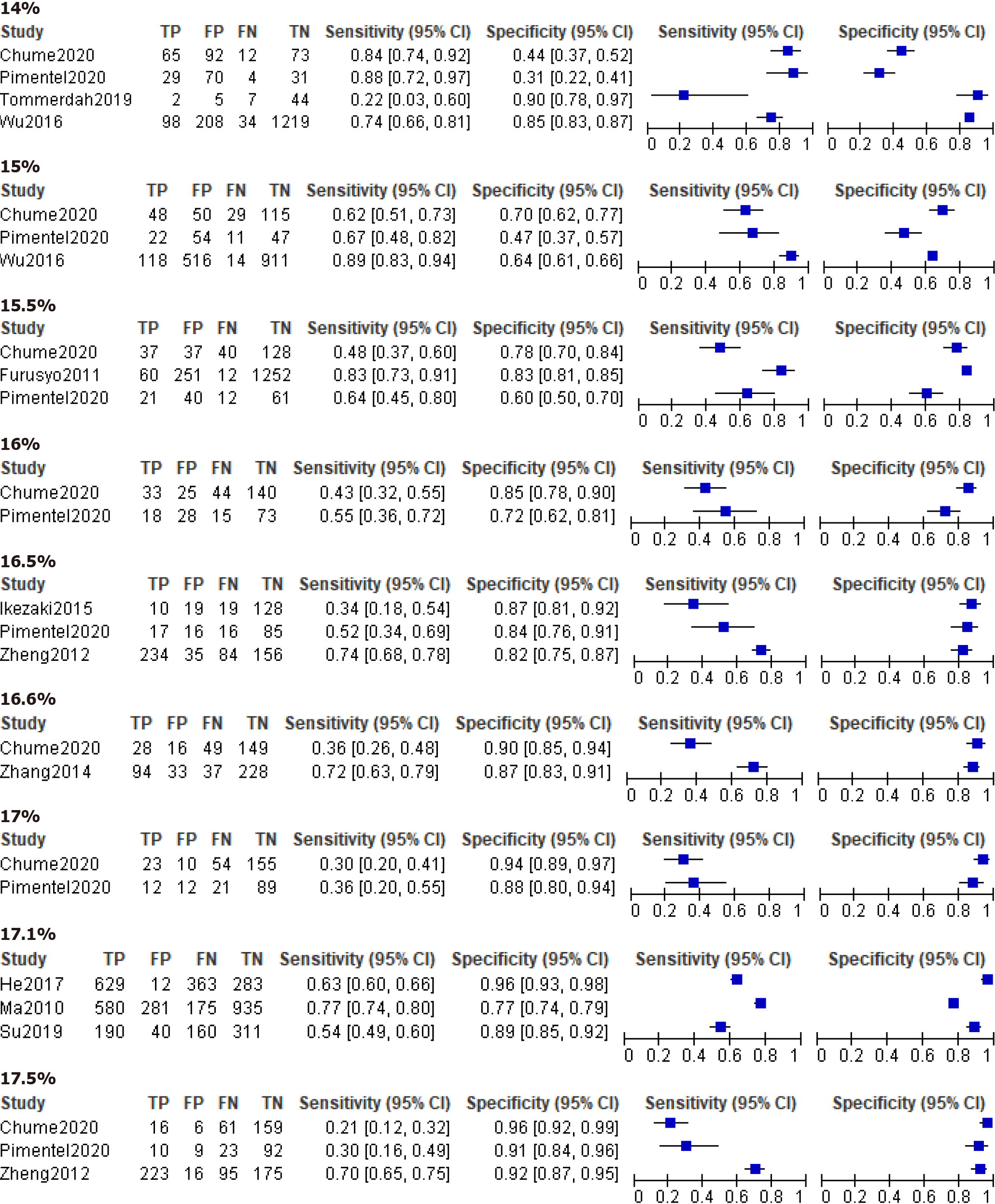
The forest plots of GDM and non-GDM were plotted with TP, FP, TN, FN values, sensitivity, and specificity combined with 95%CI extracted or calculated from original studies (Figure 4). Estimating GA test accuracy for the diagnosis of GDM by paired sensitivity and specificity or SROC was limited because of the restricted number of studies. We found that the average cut-off values reported in these GDM studies were much lower than those for another type of non-GDM.
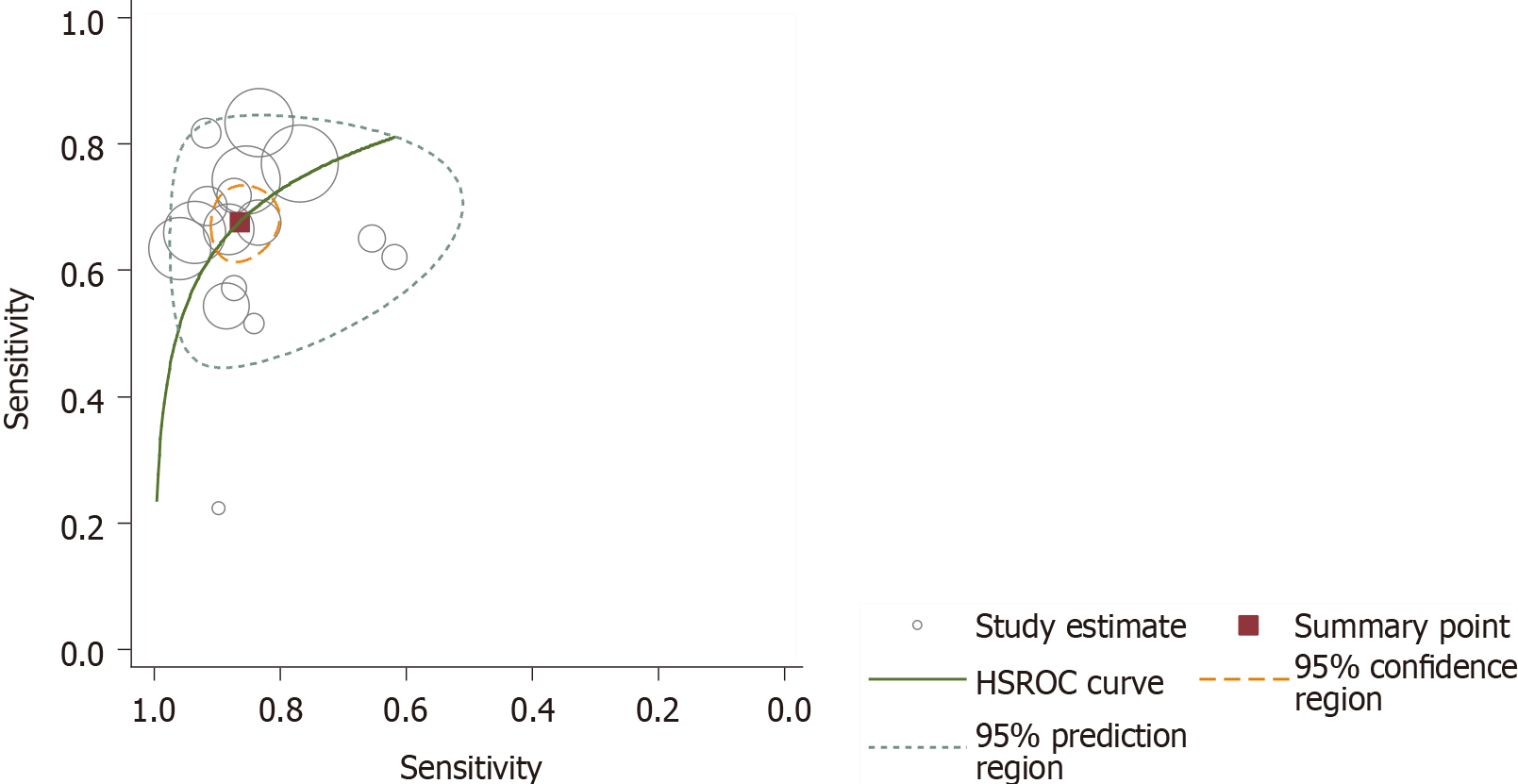
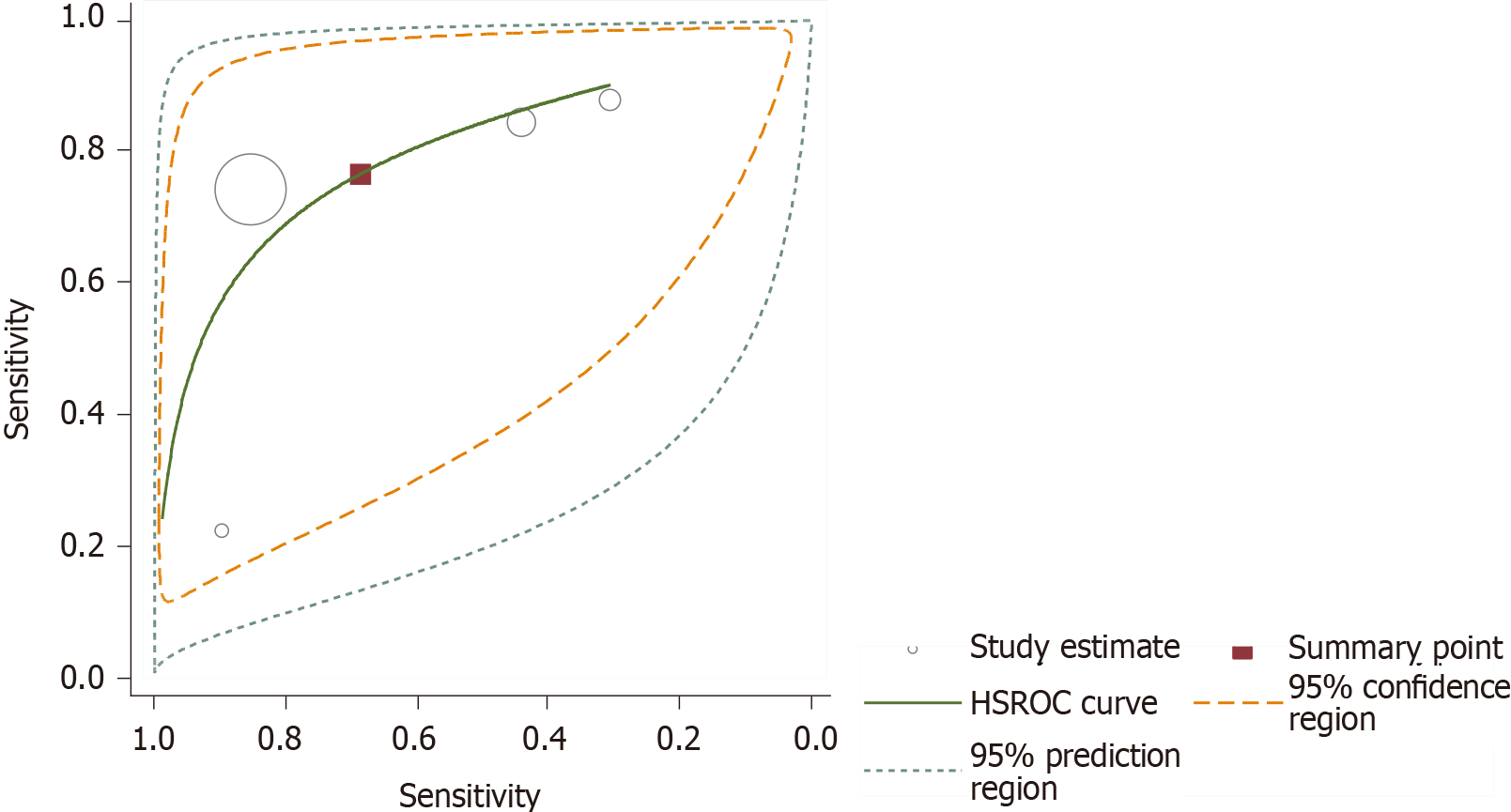
Since almost all studies reported various thresholds for the diagnosis of non-GDM, test accuracy is summarized as the SROC curve in the HSROC model (Figure 5). We avoided estimating the summary sensitivity and specificity related to an unspecified average threshold, which is clinically unhelpful. Hence, we estimated the SROC curve with the average location parameter lambda of 2.354 (95%CI: 2.002, 2.707) and scale parameter beta of 0.163 (95%CI: -0.614, 0.288). These two parameters can help us to understand the trade-off between sensitivity and specificity; if one value increases, the other decreases as the positive threshold is altered. Substantial heterogeneity existed among the non-GDM studies, as shown in the SROC plot, where the 95% prediction region is much larger than the 95% confident region. However, the heterogeneity analysis by means of meta-regression is not feasible, as few studies were available after the pre-planned subgroup analysis. Notably, one study (Tommerd et al) on CFRD reported a substantially low sensitivity. It was found that when studies on CFRD or the three on special types of DM were excluded, the difference between the 95% prediction region and 95% confident region did not show any changes. When we included GDM, the difference between the 95% prediction region and 95% confident region was greater than the earlier results. This suggests that the type of diabetes may remain as a source of heterogeneity. In the same way, when we included different BMIs and age groups, we found that they may also be the sources of heterogeneity, which should be supported by further rigorous statistical analysis.
Fortunately, four studies reported data with a common GA cut-off value of 14.0%. Figure 6 presents the forest plot of GA with a cut-off value of 14.0% for non-GDM diagnosis. Pooled sensitivity of the predicting DM was found to be 0.766 (95%CI: 0.539, 0.901) and pooled specificity was 0.687 (95%CI: 0.364, 0.894). The pooled diagnostic odds ratio was 7.176 (95%CI: 2.810, 18.324), and the AUC of the SROC was 0.80 (95%CI: 0.76, 0.83), which were calculated using the STATA 14.0 bivariate model. As shown in Figure 7, heterogeneity appeared less marked, and sensitivity analysis and meta-regression to find the source of heterogeneity were limited due to a smaller number of studies.
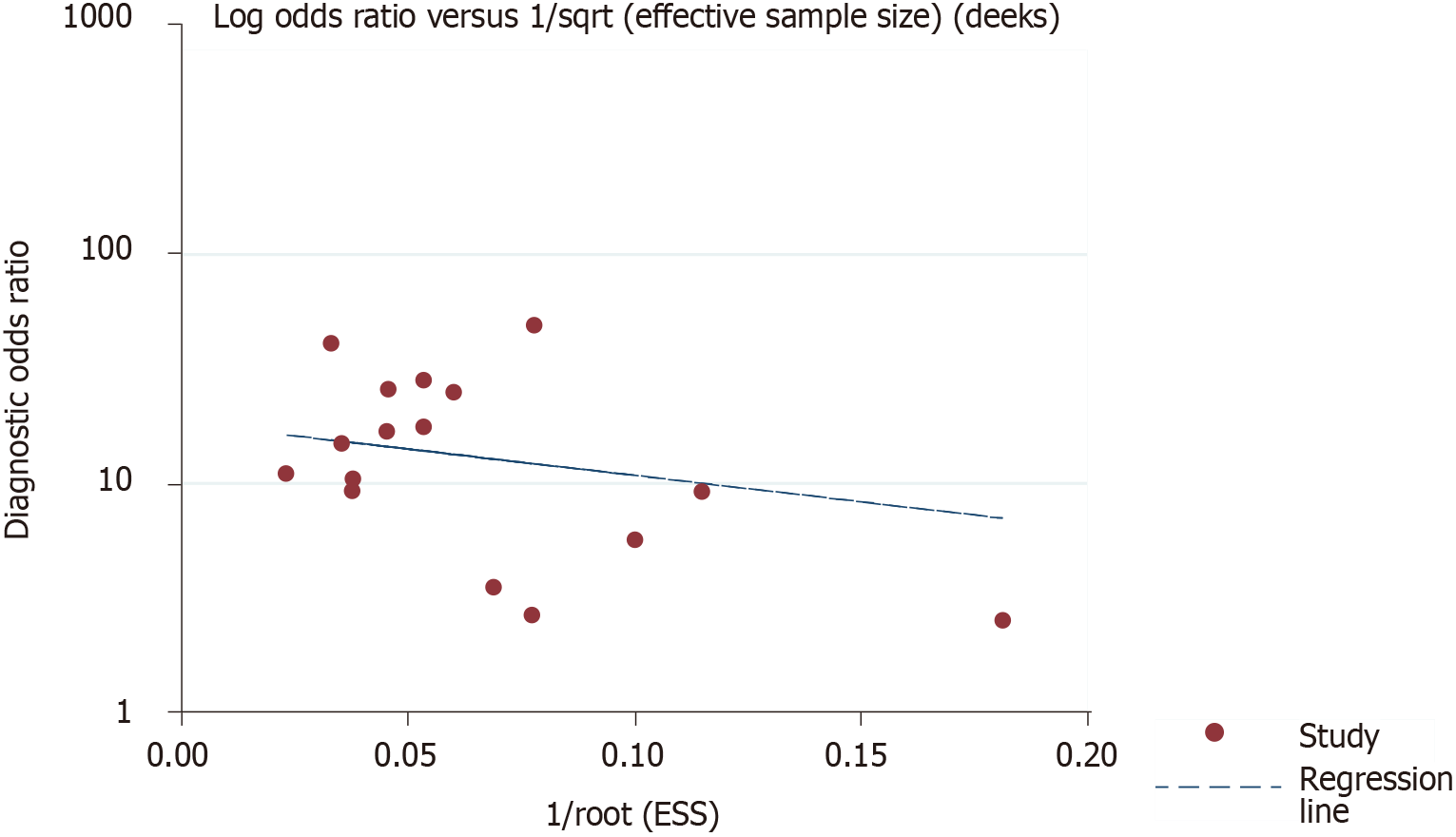
The other 12 studies reported data using cutoffs other than 14.0%. The forest plots show the sensitivity and specificity of these studies, which reported a common cutoff (Figure 6). After visual assessment, the largest Youden index may occur when the cutoff value of GA is about 15.5%. Deek’s funnel plot in Figure 8 displays a symmetrical shape with P = 0.548, which was calculated using STATA 14.0 software and identified with no publication bias in the quantitative synthesized studies.
DM is a multifactorial metabolic disorder that is diagnosed by clinical examination and laboratory tests, which are also useful for prognosis, treatment, and follow-up. Herein, our objective was to assess the utility of GA in the diagnosis of DM.
Our research included 19 studies, 3 on GDM (including 894 participants in a systematic review), and a meta-analysis of 16 non-GDM studies (consisting of 11982 subjects). Most of the studies identified using the QUADAS-2 quality assessment tool had high quality and showed a low risk of bias.
Considering the importance of early diagnosis of non-DM and GDM to delay or even interrupt the progression of complications, we must emphasize less false negative results, which means that greater sensitivity over specificity is applaudable. As such, we achieved a maximum sensitivity of 72.2% with the lowest acceptable specificity through the estimated SROC curve. A greater heterogeneity exists within DM analysis studies and becomes less heterogeneous when GDM is excluded, indicating that DM type may be one possible source of heterogeneity. In the early studies[29,30], we discovered that BMI and age may also be sources of heterogeneity. It is pertinent to note that other possible sources of heterogeneity may exist, such as study location, diabetes duration, and smoking history, which were not investigated in the included studies.
Our analysis of four studies, which assessed GA diagnostic accuracy with a cut-off of 14.0%, provided a pooled sensitivity of 0.766 with 95%CI from 0.539 to 0.901 and pooled specificity of 0.687 with 95%CI from 0.364 to 0.894. It is assumed that in a population of 1000 to-be-diagnosed subjects with a prevalence of non-GDM of 9.3%[2], 22 patients with GDM would receive false negative results, and 284 without the disease would be falsely diagnosed. The diagnostic accuracy of cut-off 14.0% is acceptable, as missed diagnosis can lead to serious complications; thus, we emphasize early stage diagnosis. In the four studies, heterogeneity was relatively smaller, and there is no evidence that heterogeneity is due to mere systematic error. The uncertainty in this group is significant with a wide 95%CI, which limits the application of pooled estimates in clinical practice. Moreover, sensitivity analysis and investigations of heterogeneity in this review were limited because of the small number of included studies.
This paper presents the first systematic review and meta-analysis on the accuracy of GA for DM, including several strengths and weaknesses. Electronic databases and clinical trial websites were systematically searched for relevant articles published or ongoing and registered trials. We used a rigorous, evidence-based process to develop QUADAS-2 from the widely used QUADAS tool[36]. We employed the bivariate model to estimate the diagnostic value of GA at a cut-off of 14.0%, which directly indicates that a correlation might exist between the estimates of sensitivity and specificity within and between the studies. The HSROC model was applied to estimate the SROC curve regarding the studies that reported different threshold values. The results account for the correlation between sensitivity and specificity across the studies as their functional relationship varies with the threshold values within each study.
The major limitations of this review are described as follows. First, bias may have been introduced since the included studies were written in English or Chinese, which may veil negative results and may cause the identified thresholds to optimize test accuracy. Second, the primary studies failed to report several essential details, such as subject selection, severity of the disease in the participants, and the procedures used to measure the index test. We were also unable to ascertain whether the various laboratory methods for the determination of GA levels were relatively uniform across the different studies, as there is no internationally recognized authoritative reference measurement procedure for GA to date. Third, the sources of heterogeneity among the studies cannot be rigorously and statistically analyzed, which reduces our confidence in the pooled estimates.
In terms of patient selection, most of the studies do not provide detailed information, leading to unpredictable bias. For example, the severity of the disease will have an impact on sensitivity, and the range of differential diagnoses present in non-diseased populations will affect the specificity. The GA detection methods included in the review may vary as differences between different diagnostic centers and laboratories in the interpretation of tests will introduce variability. In addition, application of the GA methods may be inhibited by reproducibility and calibration issues. The definition of the target disorder in the review question is also slightly different from the definition adopted in the included studies: Postprandial blood glucose was not part of the reference standard in minority studies.
Several limitations for clinical practice are attributed to the great uncertainty of the GA cut-off value and significant heterogeneity towards non-GDM. Although GA reveals a moderate accuracy for the diagnosis of non-GDM, the combination of FPG ≥ 7.0 mmol/L and/or HbA1c ≥ 6.5%GA had a higher sensitivity, and the rate of missed diagnoses was lower than that for the detection of GA[42,45,47]. In addition, clinical use of the GA detection methods is restricted to disease or comorbidities that increase albumin metabolism and decrease its half-life. Therefore, GA should be used as an additional test rather than an alternative to HbA1c or OGTT, and its use as the sole DM diagnostic test should be interpreted with caution to assure the correct classification of diabetic individuals.
Future studies are highly recommended to improve the diagnostic accuracy of GA for GDM and combinational measurement of GA and other assays with homogenous and multi-national/ethnic subjects. Authors should adhere to the recognized guidelines for reporting DTA studies, such as the Standards for Reporting of Diagnostic Accuracy Studies (STARD) initiative[56], and avoid selecting thresholds in a data-driven manner to optimize test accuracy. Besides, further studies are needed to unify international standards and optimize GA detection methods.
Glycated albumin (GA), the non-enzymatic glycation product of albumin in plasma, was first introduced as a glycemic marker in the beginning of the 21st century. GA is not affected by hemoglobin levels and reflects glycemic status over a shorter period (2-4 wk) as compared to HbA1c measurements. GA has been verified for various aspects in the management of diabetes mellitus (DM) in clinical chemistry.
This study is the first systematic review and meta-analysis on the diagnostic accuracy of GA for DM. GA may contribute as an intermediate glucose index in the current DM diagnostic system.
Our main purpose was to summarize and assess the diagnostic data to evaluate the suitability of GA in the diagnosis of DM.
The Quality Assessment of Diagnostic Accuracy Studies-2 tool was applied for the assessment of quality. The bivariate model was used to pool sensitivity and specificity, and the hierarchical summary receiver operator characteristic curve (HSROC) model was utilized to estimate the summary receiver operating characteristics curve (SROC). The results account for the correlation between sensitivity and specificity across the studies, while the HSROC model also considered the variations between the functional relationship and thresholds in each study.
The average cut-off values of GA reported for gestational diabetes mellitus (GDM) diagnosis were much lower than those for non-GDM. Diagnosing DM with a circulating GA cut-off of 14.0% had a summary sensitivity of 0.766 (95%CI: 0.539, 0.901), specificity of 0.687 (95%CI: 0.364, 0.894), and area under the curve (AUC) of 0.80 (95%CI: 0.76, 0.83) for SROC. The estimated SROC at different GA cut-off values for non-GDM exhibited that the average location parameter lambda was 2.354 (95%CI: 2.002, 2.707) and the scale parameter beta was -0.163 (95%CI: -0.614, 0.288).
GA should be used as an additional test rather than an alternative to HbA1c or OGTT, considering its moderate accuracy in diagnosing non-GDM. Its use as the sole DM diagnostic test should be interpreted with caution to assure the correct classification of diabetic individuals.
Further research on the diagnostic accuracy of GA for GDM and combinational measurements of GA with other assays has been suggested. Besides, it is pertinent for researchers to unify international standards and optimize GA detection methods.
The authors would like to thank Min Zhang for her statistical advice during the preparation of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Song A S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Goyal R, Jialal I. Diabetes Mellitus Type 2. 2021 Sep 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. [PubMed] [Cited in This Article: ] |
| 2. | International Diabetes Federation. Diabetes Atlas Ninth Edition 2019. [cited 20 February 2021]. Available from: https://diabetesatlas.org/en/resources/. [Cited in This Article: ] |
| 3. | Rodríguez-Gutiérrez R, Quintanilla-Flores DL, Portillo-Sanchez P. McMaster, Hinojosa-Amaya JM, Morey-Vargas OL, Montori VM, Sieradzki J, Płaczkiewicz-Jankowska E. McMasterTextbook of Internal Medicine:Diabetes Mellitus. Kraków: MedycynaPraktyczna. Last Updated: 2019 [cited 20 February 2021]. Available from: https://empendium.com/mcmtextbook/chapter/B31.II.24. [Cited in This Article: ] |
| 4. | Herman WH, Ye W, Griffin SJ, Simmons RK, Davies MJ, Khunti K, Rutten GE, Sandbaek A, Lauritzen T, Borch-Johnsen K, Brown MB, Wareham NJ. Early Detection and Treatment of Type 2 Diabetes Reduce Cardiovascular Morbidity and Mortality: A Simulation of the Results of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe). Diabetes Care. 2015;38:1449-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Kim W, Park SK, Kim YL. Gestational diabetes mellitus diagnosed at 24 to 28 weeks of gestation in older and obese Women: Is it too late? PLoS One. 2019;14:e0225955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167:1545-1551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33:562-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 485] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 8. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1853] [Cited by in F6Publishing: 2032] [Article Influence: 338.7] [Reference Citation Analysis (0)] |
| 9. | Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies--cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9:e111463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 11. | Juraschek SP, Steffes MW, Miller ER 3rd, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of Community-Based Reference Intervals for Fructosamine, Glycated Albumin, and 1,5-Anhydroglucitol. Clin Chem. 2018;64:843-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Yazdanpanah S, Rabiee M, Tahriri M, Abdolrahim M, Rajab A, Jazayeri HE, Tayebi L. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: A comprehensive review. Crit Rev Clin Lab Sci. 2017;54:219-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153-2163. [PubMed] [Cited in This Article: ] |
| 15. | Desouza CV, Rosenstock J, Zhou R, Holcomb RG, Fonseca VA. Glycated albumin at 4 weeks correlates with A1C levels at 12 weeks and reflects short-term glucose fluctuations. Endocr Pract. 2015;21:1195-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Ueda Y, Matsumoto H. Recent topics in chemical and clinical research on glycated albumin. J Diabetes Sci Technol. 2015;9:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Rendell M, Kao G, Mecherikunnel P, Petersen B, Duhaney R, Nierenberg J, Rasbold K, Klenk D, Smith PK. Aminophenylboronic acid affinity chromatography and thiobarbituric acid colorimetry compared for measuring glycated albumin. Clin Chem. 1985;31:229-234. [PubMed] [Cited in This Article: ] |
| 18. | Ikeda K, Sakamoto Y, Kawasaki Y, Miyake T, Tanaka K, Urata T, Katayama Y, Ueda S, Horiuchi S. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA). Clin Chem. 1998;44:256-263. [PubMed] [Cited in This Article: ] |
| 19. | Morais MP, Mackay JD, Bhamra SK, Buchanan JG, James TD, Fossey JS, van den Elsen JM. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics. 2010;10:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Bohli N, Meilhac O, Rondeau P, Gueffrache S, Mora L, Abdelghani A. A facile route to glycated albumin detection. Talanta. 2018;184:507-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Yasukawa K, Abe F, Shida N, Koizumi Y, Uchida T, Noguchi K, Shima K. High-performance affinity chromatography system for the rapid, efficient assay of glycated albumin. J Chromatogr. 1992;597:271-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther. 2010;14:49-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Paleari R, Bonetti G, Callà C, Carta M, Ceriotti F, Di Gaetano N, Ferri M, Guerra E, Lavalle G, Cascio CL, Martino FG, Montagnana M, Moretti M, Santini G, Scribano D, Testa R, Vero A, Mosca A. Multicenter evaluation of an enzymatic method for glycated albumin. Clin Chim Acta. 2017;469:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Hashimoto K, Tanikawa K, Nishikawa J, Chen Y, Suzuki T, Koga M. Association of variation range in glycated albumin (GA) with increase but not decrease in plasma glucose: implication for the mechanism by which GA reflects glycemic excursion. Clin Biochem. 2015;48:397-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Lu JM, Ji LN, Li YF, Li QM, Lin SS, Lv XF, Wang L, Xu Y, Guo XH, Guo QY, Ma L, Du J, Chen YL, Zhao CL, Zhang QL, She QM, Jiao XM, Lu MH, Pan RQ, Gao Y. Glycated albumin is superior to glycated hemoglobin for glycemic control assessment at an early stage of diabetes treatment: A multicenter, prospective study. J Diabetes Complications. 2016;30:1609-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Nathan DM, McGee P, Steffes MW, Lachin JM; DCCT/EDIC Research Group. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63:282-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Liu L, Zeng L, Sang D, Lu Z, Shen J. Recent findings on fulminant type 1 diabetes. Diabetes Metab Res Rev. 2018;34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Zhou Q, Shi DB, Lv LY. The establishment of biological reference intervals of nontraditional glycemic markers in a Chinese population. J Clin Lab Anal. 2017;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Matsha TE, Korf M, Erasmus RT, Hoffmann M, Mapfumo C, Smit F, Zemlin AE. Reference interval determination for glycated albumin in defined subgroups of a South African population. Ann Clin Biochem. 2019;56:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Bellia C, Zaninotto M, Cosma C, Agnello L, Bivona G, Marinova M, Lo Sasso B, Plebani M, Ciaccio M. Clinical usefulness of Glycated Albumin in the diagnosis of diabetes: Results from an Italian study. Clin Biochem. 2018;54:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Hsu P, Ai M, Kanda E, Yu NC, Chen HL, Chen HW, Cheng MH, Kohzuma T, Schaefer EJ, Yoshida M. A comparison of glycated albumin and glycosylated hemoglobin for the screening of diabetes mellitus in Taiwan. Atherosclerosis. 2015;242:327-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Wu WC, Ma WY, Wei JN, Yu TY, Lin MS, Shih SR, Hua CH, Liao YJ, Chuang LM, Li HY. Serum Glycated Albumin to Guide the Diagnosis of Diabetes Mellitus. PLoS One. 2016;11:e0146780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1683] [Cited by in F6Publishing: 1635] [Article Influence: 272.5] [Reference Citation Analysis (0)] |
| 36. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6953] [Cited by in F6Publishing: 8263] [Article Influence: 635.6] [Reference Citation Analysis (0)] |
| 37. | Lang K, Wang Y, Li X, Shi C, Xiao C, Hu J. Application value of glycated albumin and fasting plasma glucose in gestational diabetes. Yixue Jianyan Yu Linchuang. 2019;29:7-10. [DOI] [Cited in This Article: ] |
| 38. | Liu H, Yang J, Zhang J, Xu D, Lu Q. Predictive value of glycated albumin and body mass index in second trimester to gestational diabetes. Sichuan Yixue. 2020;41:803-806. [DOI] [Cited in This Article: ] |
| 39. | Zhuang J, Wang H, Dai L, Li B, Se G. Diagnostic value of glycosylated albumin in the second trimester of pregnancy for normal fasting glucose in gestational diabetes mellitus. Nanjing Yike Daxue Xuebao. 2019;39:559-562. [DOI] [Cited in This Article: ] |
| 40. | Pimentel AL, Hernandez MK, Freitas PAC, Chume FC, Camargo JL. The usefulness of glycated albumin for post-transplantation diabetes mellitus after kidney transplantation: A diagnostic accuracy study. Clin Chim Acta. 2020;510:330-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Wang L, Gao Y, Wang G, Shi KW, Wang B, Li YJ, Gao W, Jing GX. The predictive value of glycated albumin in renal transplant recipients with post-transplant diabetes mellitus. Xi'an Jiaotong Daxue Xuebao (Medical Sciences). 2020;41:108-113. [DOI] [Cited in This Article: ] |
| 42. | Chume FC, Kieling MH, Correa Freitas PA, Cavagnolli G, Camargo JL. Glycated albumin as a diagnostic tool in diabetes: An alternative or an additional test? PLoS One. 2019;14:e0227065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Zemlin AE, Barkhuizen M, Kengne AP, Erasmus RT, Matsha TE. Performance of glycated albumin for type 2 diabetes and prediabetes diagnosis in a South African population. Clin Chim Acta. 2019;488:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Tommerdahl KL, Brinton JT, Vigers T, Nadeau KJ, Zeitler PS, Chan CL. Screening for cystic fibrosis-related diabetes and prediabetes: Evaluating 1,5-anhydroglucitol, fructosamine, glycated albumin, and hemoglobin A1c. Pediatr Diabetes. 2019;20:1080-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Su H, Ma XJ, Ying LW, He XX, Zhu W, Tang JL, Wang YF, Bao YQ, Zhou J. Efficiency comparison of fasting plasma glucose combined with 1,5-anhydroglucitol and combined with glycated albumin in diabetes mellitus screening. Shanghai Jiaotong Daxue Xuebao (Medical Science). 2019;39:1077-1082. [DOI] [Cited in This Article: ] |
| 46. | Su H, Tang J, Ma X, He X, Ying L, Wang Y, Bao Y, Zhou J. Postload Glycated Albumin as an Alternate Measure for Diabetes Screening in a Chinese Population. J Diabetes Res. 2018;2018:7932528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | He X, Ying L, Ma X, Shen Y, Su H, Peng J, Wang Y, Bao Y, Zhou J, Jia W. An additional measurement of glycated albumin can help prevent missed diagnosis of diabetes in Chinese population. Clin Chim Acta. 2017;475:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Ikezaki H, Furusyo N, Ihara T, Hayashi T, Ura K, Hiramine S, Mitsumoto F, Takayama K, Murata M, Kohzuma T, Ai M, Schaefer EJ, Hayashi J. Glycated albumin as a diagnostic tool for diabetes in a general Japanese population. Metabolism. 2015;64:698-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Hwang YC, Jung CH, Ahn HY, Jeon WS, Jin SM, Woo JT, Cha BS, Kim JH, Park CY, Lee BW. Optimal glycated albumin cutoff value to diagnose diabetes in Korean adults: a retrospective study based on the oral glucose tolerance test. Clin Chim Acta. 2014;437:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Zhang T, He H, Yang HL, Huang HJ, Zhang MF, An ZM, Li SQ. [Study of glycated albumin cut-off point in diabetes mellitus and impaired glucose regulation]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:274-277, 298. [PubMed] [Cited in This Article: ] |
| 51. | Zheng H, Zhou XM, Ma LF, Wang BA, Mu MY. Value of HbA1c and glucoprotein for diagnosis of type 2 diabetes mellitus: A comparative study. Jiefangjun Yixueyuan Xuebao. 2012;33:158-160. [Cited in This Article: ] |
| 52. | Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011;54:3028-3036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Ma XJ, Pan JM, Bao YQ, Zhou J, Tang JL, Li Q, Xiang KS, Jia WP. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010;37:974-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract. 1989;7:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Rodriguez-Capote K, Tovell K, Holmes D, Dayton J, Higgins TN. Analytical evaluation of the Diazyme glycated serum protein assay on the siemens ADVIA 1800: comparison of results against HbA1c for diagnosis and management of diabetes. J Diabetes Sci Technol. 2015;9:192-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF; STARD Group. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin Chem. 2015;61:1446-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 373] [Article Influence: 41.4] [Reference Citation Analysis (0)] |