Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8425
Peer-review started: February 9, 2021
First decision: March 30, 2021
Revised: April 5, 2021
Accepted: August 11, 2021
Article in press: August 11, 2021
Published online: October 6, 2021
The hepatic artery (HA) is one of the most threatened vascular structures during hepatopancreatobiliary (HPB) surgeries and interventional procedures. It can be affected by many clinical pictures, especially tumors, due to its anatomical position and neighborhood.
To reveal the evolution and recent developments in the management of HA traumas in the light of the literature.
In this article, 100 years of MEDLINE (PubMed) literature and articles including cases and series of HA injuries were reviewed, and the types of injury occurrence, treatment, and related complications and their management were compiled.
The risk of HA injury increases during cholecystectomies and pancreatoduodenectomies, among the most common operations. HA anatomy shows anomalies in approximately 15%-25% of the cases, further increasing this risk. The incidence of HA injury is not precisely known. Approaches that have evolved in recent years in managing patients with HA injury (laceration, transection, ligation, resection) with severe morbidity and mortality risk are reviewed in light of the current literature.
In conclusion, complications and deaths due to HA injury are less common today. The risk of complications increases in patients with hemodynamic instability, jaundice, cholangitis, and sepsis. Revealing the variations in the preoperative radiological evaluation will reduce the risks. In cases where HA injury is detected, arterial flow continuity should be tried to maintain with primary anastomosis, arterial transpositions, or grafts. In cases where bile duct injury develops, patients should be directed to HPB surgery centers, considering the possibility of accompanying HA injury. Large-scale and multicentric studies are needed to understand better the early and long-term results of HA ligation and determine preventive procedures.
Core Tip: The hepatic artery (HA) is one of the most threatened vascular structures during hepatopancreatobiliary surgeries and interventional procedures. Complications and deaths due to HA injury are less common today. The risk of complications increases in patients with hemodynamic instability, jaundice, and cholangitis. Revealing the variations in the preoperative radiological evaluation will reduce the risks. In cases where HA injury is detected, arterial flow continuity should be tried to be maintained with primary anastomosis, arterial transpositions, or grafts. In cases where bile duct injury develops, patients should be directed to hepatopancreatobiliary surgery centers, considering the possibility of accompanying HA injury. Large-scale and multicentric studies are needed to understand better the early and long-term results of HA ligation and determine preventive procedures.
- Citation: Dilek ON, Atay A. Dealing with hepatic artery traumas: A clinical literature review. World J Clin Cases 2021; 9(28): 8425-8440
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8425.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8425
The hepatic artery (HA) is one of the most threatened vascular structures during hepatopancreatobiliary (HPB) surgeries and interventional procedures. HA can be affected by many clinical pathologies, especially tumors, due to its anatomical position and neighborhood. HA injury was one of the deadliest complications in the 1940s. In Edgecombe and Gardner's studies, it was found that 40 HA ligations were reported until 1950, and mortality developed in 50%-75% of them. They reported that HA was ligated during artery aneurysm, bile duct tumor, stomach tumor, and pancreatic tumor surgeries[1,2]. Although significant improvements in complications and mortality rates due to HA injuries have been detected in recent years, it continues to cause severe morbidity and mortality[3].
It has been reported that almost all animals (rats, rabbits, pigs) who underwent artery ligation experimentally died[4,5]. In experimental studies with dogs, it was reported that dogs were much more resistant to HA ligation. Especially in dogs that were given antibiotics (penicillin), it was better tolerated[5-7]. The use of antibiotics reduces the damage to the liver. Unlike other animals, it has been found that dogs have too much collateral in their liver. If collaterals are ligated, the dogs die within 24 h[5]. In the same period, it was reported that humans' average survival is 9-10 d due to artery ligation[1,2].
HA anomalies can also increase the risk of injury. HA anomalies are seen in 15%-25% of the population[2,3,8]. The most common anomaly is the replaced right HA (rRHA) anomaly. In the studies conducted, there is a common belief that more HA lacerations occur in patients with abnormalities[3,9,10].
In autopsy studies, it has been stated that deaths seen as a result of HA injury are mainly due to liver necrosis. Necrosis that occurs in the liver is diffuse or patchy[2,3,11]. Better results are obtained today with a better understanding of liver physiology, antibiotics, and improved intensive care conditions.
In this study, we aimed to examine the problems related to HA injuries, ligations, and resections encountered during our HPB operations, the approaches we applied, and the prevention methods.
In this article, 100 years of MEDLINE (PubMed) literature was reviewed. A clinical approach algorithm was developed by reviewing articles, including cases and series of HA injuries. In the study, keywords containing "hepatic artery" and (trauma or injury or resection or ligation or avulsion or transection or reconstruction) and their various combinations were researched.
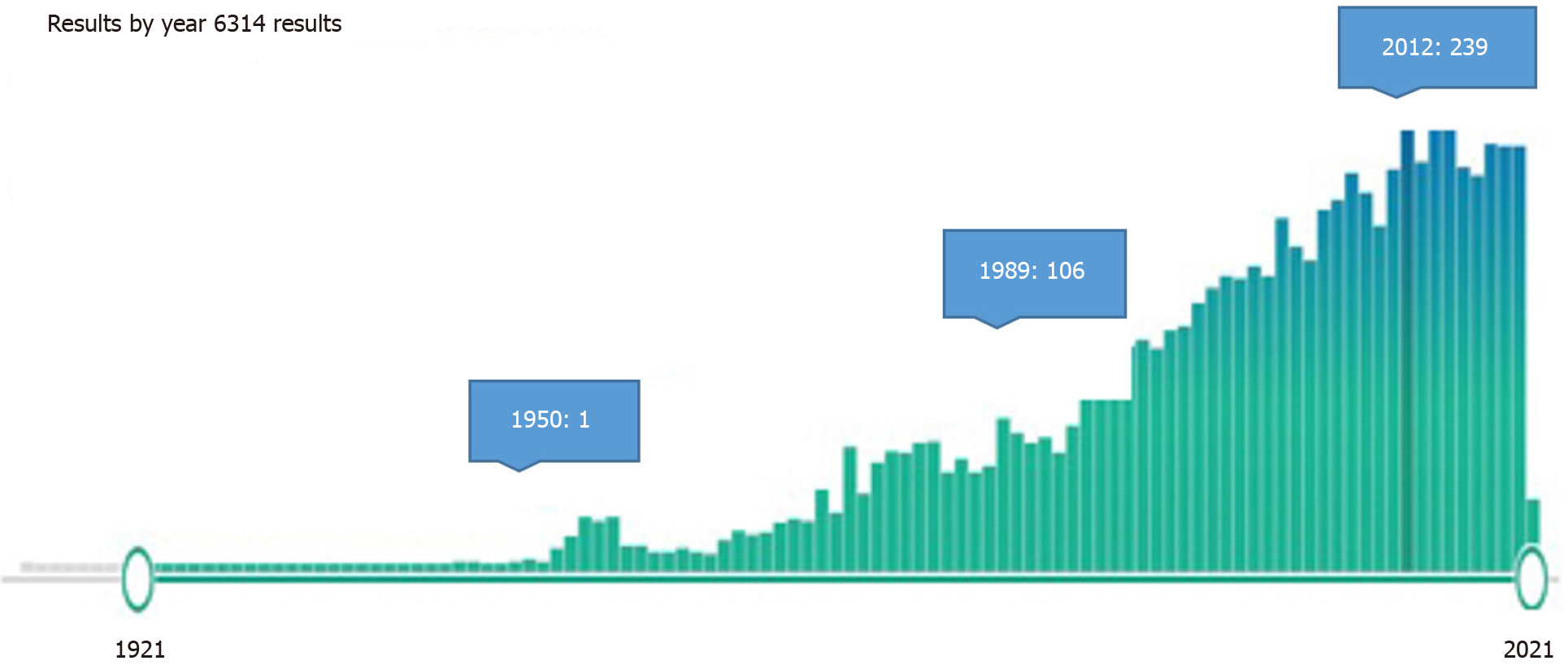
Articles on HA transection and reconstruction, a challenging stage of liver transplantation, were not included in the study. Planned ligation, resection, and reconstructions for HA aneurysms were also briefly discussed. HA embolization has found many more clinical applications with the developing technological applications, and more studies have been reported in recent years (Figure 1). Here, therapeutic HA embolization is also mentioned because of its similarity to liver damage seen in HA traumas.
It was found that there were 6314 articles as a result of the Medline research. While one to two articles were published annually in the first 50 years, this number has increased gradually in the last 50 years and reached 109-237 articles per year (Figure 1). There are 1555 articles with the keywords "hepatic artery injury" or "hepatic artery trauma" and 468 articles with the word "hepatic artery ligation". In the first half of the century, it was found that HA traumas were mostly applied with unintentional ligations during gallbladder and stomach surgeries and in patients who underwent planned ligation for HA aneurysm. Fifty-seven studies with the phrase "hepatic artery resection" were identified; most of them conducted in the last 20 years. "Hepatic artery embolization" was the subject of 406 studies, and most of them were published in the previous 2 decades.
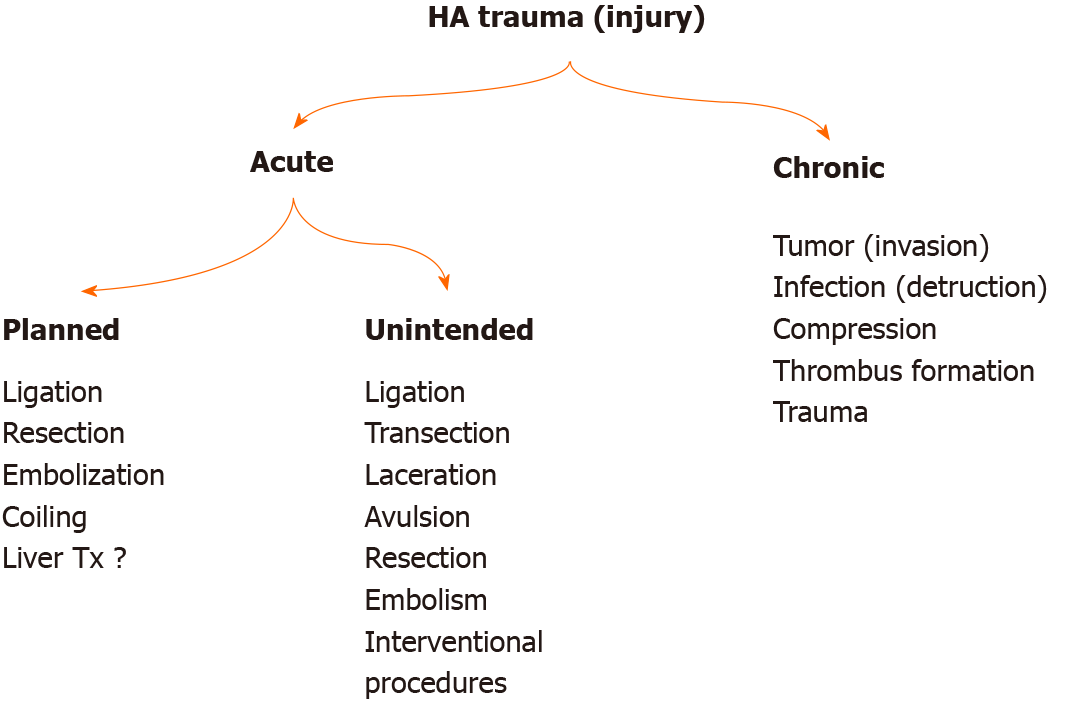
HA can be affected by many pathological conditions, especially tumors, due to its anatomical position and neighborhood (Figure 2). It is one of the most threatened vascular structures during HPB surgeries. The risk of injury is higher during Laparoscopic cholecystectomy (LC), pancreaticoduodenectomy (PD), and bile duct surgeries, which are among the most commonly performed operations in the abdomen[3,10,12,13]. HA can rarely be injured during stab wounds. HA has become an essential tool for diagnosis and treatment, and injuries occur in various forms depending on interventional procedures[14]. The incidence of HA injury is not precisely known. There is minimal data on this subject in the literature.
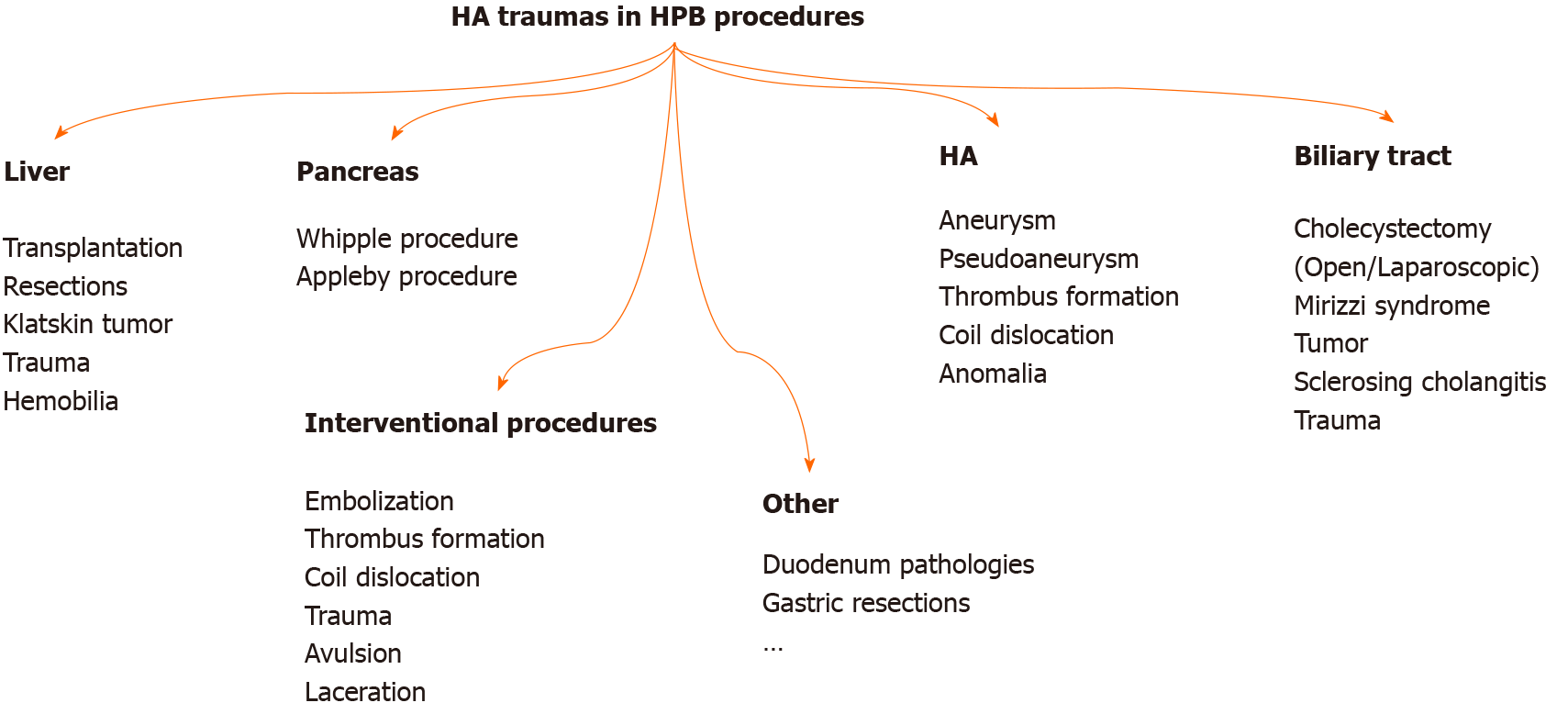
HA is injured mainly during HPB surgeries (Figure 3). However, injury can also occur during interventional procedures and with penetrant tools or blunt abdominal injuries. HA injury may develop with laceration, transection, avulsion, and unintended ligation/resection. Arterial blood flow can also be impaired with planned embolization, ligation, and resection of HA. Damage and invasion of HA by path
The degree of liver damage after HA trauma depends on many factors (Table 1)[6,7,15-17]. Some of these are; 1-artery anatomy, 2-location of injury/ligation, 3-tissue bacteriology, 4-portal vein capacity, 5-collateral system and variations, 6-hemodynamic stability and complications, and 7-duration of HA trauma. The location of the injury/ligation is one of the most critical factors. Naturally, the blood supply tries to be preserved by prehepatic, intrahepatic, and perihepatic collateral mech
| Factors affecting the extent of liver damage in hepatic artery trauma |
| Artery anatomy |
| Location of injury |
| Portal vein capasity |
| Collateral system and variations |
| Hemodynamic stability |
| Complications/comorbidities |
| Duration of HA trauma (injury invasion) |
| Dimensions of the operation/intervention |
| Cholangitis (sepsis) ? |
| Tissue bacteriology |
| Hyperbilirubinemia |
Damage to the liver resulting from impaired hepatic artery blood flow for any reason has similar characteristics. The fact that HA injury is acute or chronic is another factor affecting the outcome. Many studies have been reported without reconstruction following resections in patients with the HA invasion by the tumor[19,20]. The collateral system, which occurs in cases where arterial occlusion develops slowly, is thought to reduce/prevent liver damage after resection. However, many factors such as the size of the operation, accompanying procedures, patient's comorbidity, hemodynamic stability, cholangitis (sepsis), jaundice, and liver residue may affect the degree of liver damage and the clinical picture[16,20,21].
The frequency of vascular injury during LC was reported as 0.25%, and the frequency of HA injury was reported as 0.06% in the literature[10,12,13]. In other words, 10%-47% of the patients who develop bile duct injury can also develop HA injury[10,12,13,15]. Interestingly, it is estimated that right HA injury is more than predicted in the evaluations[10,15,22,23]. This is because LC is a widespread and ubiquitous surgery. Also, more surgical interventions are performed for biliary tract and hilar region malignancies than in the past. A publication by Gordon-Taylor[11] in 1943 reported that surgery in some patients who died after cholecystectomy was performed by inexperienced surgeons (amateurs); the cause of death might be clamping or ligating the RHA. In their series of 261 cases of bile duct injury developed during LC by Stewart et al[10], they found that right HA injury occurred in 84 patients. They reported that HA injury is more common in Class 3 and 4 type bile duct injuries (Stewart-Way classification). They also noted that additional new HA injuries were more likely to occur in cases in which the primary surgeon tried to repair them. The risk of injury was much lower in experienced centers. Interestingly, they found 11% of RHA-injured cases in the series developed liver ischemia. Preoperative or intraoperative Doppler ultrasonography may help understand whether artery revision is required in patients undergoing reconstruction due to bile duct injury. In the postcholecystectomy biliary stricture series of 55 cases by Alves et al[15], they found HA injury in 47% of the cases, mostly RHA and rRHA. Li et al[12] found that HA injury developed in 10 patients in their series of 60 postcholecystectomy biliary strictures.
The incidence of HA injury during PD ranges from 0.1% to 4.4%. During resections in patients with chronic pancreatitis or locally advanced tumors, the risk of vascular injury increases due to intense adhesions and inflammation[12,20,24]. In large tumors, the risk of trauma may increase due to the displacement of anatomical structures and tumor invasion. Gaujoux et al[24] performed angiographies in their PD series of 545 cases to investigate postoperative ischemic conditions. They performed recon
HA anomalies are another factor that increases the risk of injury[20,25]. Intertwined anatomical relationships between the pancreas and regional vessels become more complicated with the anomaly, increasing vascular injury risk. According to Michel's classification, a typical anatomical structure (Type 1) is present in 52%-80% of cases. In cadavers and clinical trials, HA anomaly was found in 15%-25% of the cases. Shukla et al[26] stated that HA variations might show in 55%-79% of patients.
The incidence of rRHA was reported in the literature as 6.7%-19%[8,9,19,25-27]. Rubio et al[28]'s series stated that 73% of HA injury occurs in anomaly arteries. Eshuis et al[9] detected rRHA (18.8%) in 143 cases in the PD series of 758 cases and found injuries in 13 of them. At the same time, 10 patients had severe morbidity, while 1 patient died.
Accessory left HA (aLHA) incidence varies between 3%-34.2%. LHA or aLHA injury is more likely to occur during the celiac region's dissection for gastric cancer[29,30].
HA trauma (rupture, thrombus, embolus, aneurysm, fistula) may develop accidentally and dislocated coils and stents during the interventional procedures. Vascular occlusion may impair HA blood flow. Blocking of the celiac trunk with thrombus can also interrupt HA blood flow. Lacerations, avulsions, and transections may develop due to sharp or blunt traumas[14,31].
Many procedures and clinical studies in which HA was linked due to liver-derived pathologies (hepatocellular carcinoma, cirrhosis, portal hypertension, hemangioma, liver trauma, hemobilia) have been described[22,32-36]. It is predicted that by ligating HA, the amount of blood coming to the liver will decrease by 35%, whereas 95% of the blood requirement of a metastatic tumor in the liver will be reduced[32]. This effect is observed more in hypervascular metastases (hypernephroma, leiomyosarcoma, carcinoid, papillary adenocarcinoma of the pancreas)[32]. In their 19-disease invasive hepatocellular carcinoma series by Elsanousi et al[34], 13 of the patients who underwent ligation of HA on the lesion side and extrahepatic collateral divisions (HALED) received a complete response. They also reported that there were no abscesses and necrosis in the liver. However, there is not enough literature on this subject, and prospective studies are needed.
HA ligation, resection, and reconstruction can also be performed in HA aneurysm, pseudoaneurysm, abdominal aortic dissections, and HELLP syndrome[1,2,37,38]. In a meta-analysis including 374 hilar cholangiocarcinoma cases with HA resections performed by Chen et al[39], they found that the rate of R0 resection was higher in those who had HA resection. They found that mortality and morbidity were higher in the group with HA resection. However, there was no statistically significant difference in terms of complications. They also reported that the group's survival with R0 resection and HA resection was much better when combined with adjuvant chemotherapy. During Appleby or Whipple procedures performed in borderline pancreatic tumors, aggressive tumor resections and artery resections have been increasingly performed due to tumor invasion[12,20,24,40]. Kleive et al[20] performed planned HA resection in 22 (1.43%) cases in a pancreatectomy series of 1535 cases. They reported that complications developed in a total of 16 (73%) cases, 10 of them (45%) being severe (thrombosis, bleeding, stenosis, liver necrosis, bile leakage). In the PD series of 323 patients by Asano et al[19], they detected rRHA anomaly in 51 patients. They performed planned resections in eight of them, and they reported that accidental injury occurred in one. They found that liver abscess developed in only 1 case in the series without reconstruction. There was no statistical difference with other patients in terms of demographics.
When compared with the complications seen after HA trauma, it was determined that fewer complications developed after planned HA resections[19,20]. The reasons for this may be technological developments, use of antibiotics and improvements in intensive care conditions, less liver damage in hemodynamically stable patients, and the contribution of collateral networks that develop during the invasion of the HA.
Angiography, stenting, coiling, and embolization are increasingly preferred methods for diagnosing and treating patients with hemodynamically stable liver trauma. Blood supply of the liver may also be impaired in patients who have been embolized for treatment purposes (tumor, metastasis, hemangioma, aneurysm, postoperative bleeding, etc.) or due to HA trauma. Selective embolization is better tolerated. It has been reported in the literature that the procedure is generally well-tolerated, and serious complications such as liver necrosis, cholangitis, and liver abscess are much less common[14,41,42]. In the Transarterial chemoembolization series of 2300 sessions applied by Sakamoto et al[42] to 850 patients, it was reported that complications (4 liver necrosis, 5 liver abscesses, 7 cholecystitis, 20 biloma, 6 aneurysms, and others) developed in 4.4% (n = 102 procedures) of the applications. Minimally invasive intervention and preservation of hemodynamic stability may contribute to better results.
Brittain et al[16] described the striking color change in the right liver lobe as an ominous sign in a patient who developed RHA laceration during cholecystectomy in 1964. The dimension of the damage varies according to the surgical intervention and the size of the HA injury. While most isolated HA injuries heal without symptoms, 11%-76% of patients with both RHA and bile duct trauma have been reported to have ischemic damage to the liver[3,12,15,43]. HA adventitia can be damaged by excessive manipulation, traction, and compression, and the risk of developing pseudoaneurysm increases, especially in pancreatic fistula cases[9,26].
As a result of HA injury, liver abscess, liver failure, anastomosis opening, late liver atrophy, and bile duct stenosis are the most common complications[3,9,35,41,44]. In the HA injury series of 21 cases, Landen et al[3] detected in the PubMed screen complications: Liver necrosis/abscess (n = 14), liver failure (n = 3), and anastomotic dehiscence (n = 6) were reported in 16 patients (76%), 3 of which had artery variations. They also noted that 11 of the patients were re-operated, and five (24%) died.
Due to ischemia of the bile duct wall caused by HA injury, anastomotic leakage may occur in the early period, and biliary stricture may develop in the long term[15,43]. The mucosal damage due to ischemia in the bile duct mucosa heals with inflammation, and fibrosis also leads to biliary stricture. Recurrent cholangitis and hepatolithiasis can also be seen in patients with biliary stricture. In an autopsy study, stenosis in the biliary tract was found in 7% of cadavers with open cholecystectomy[10,45].
Right lobe atrophy can also be seen as a result of RHA injury or ligation. Alves et al[15] reported that in a series of 55 cases of postcholecystectomy biliary stricture after HA injury, they detected right lobe atrophy in 12 patients and performed right hepatectomy and Roux-en-Y hepaticojejunostomy.
Left lobe atrophy mainly develops due to left HA injury. Due to the high incidence of aLHA injury (36%-43%), it is accepted that liver left lobe atrophy is more common after radical gastric resections. LHA injury is less common following PD surgeries. However, aLHA and LHA injuries can be seen more during dissections for stomach tumors[15,29].
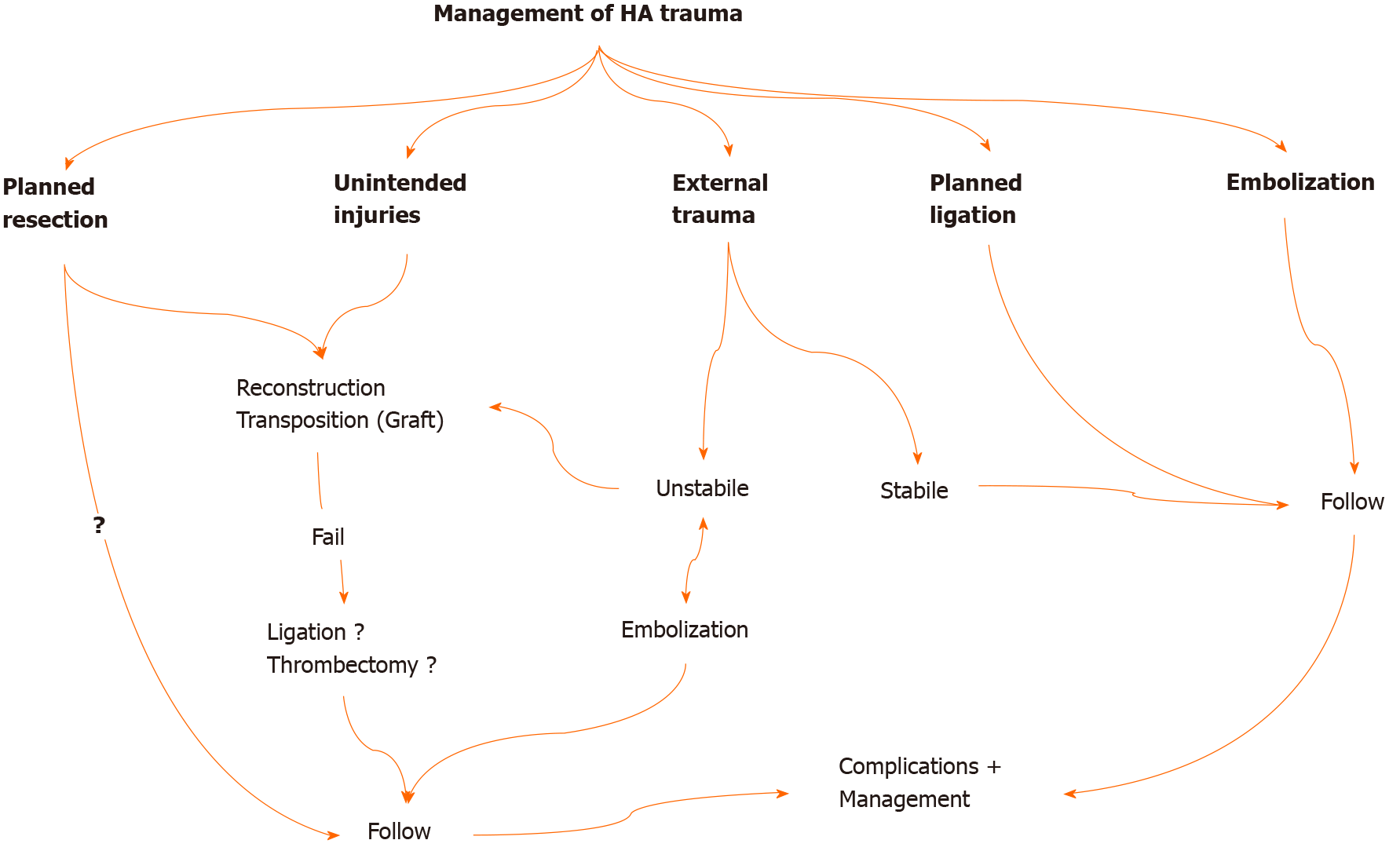
Management changes according to the type of HA injury (Figure 4). Reconstruction should be done in unintended HA traumas encountered during surgery[12,15,20,24]. If possible, reconstruction should be performed in the same session. Primary reconstruction of the laceration area should be tried first. In cases where proper HA transection develops, continuity of blood flow can be achieved by transposing the gastroduodenal artery, left gastric artery, or splenic artery. Reconstruction can also be performed using an allogeneic prosthetic graft (polytetrafluoroethylene, Dacron) or autografts (saphenous vein, gonadal vein, inferior mesenteric vein, renal vein, gastroepiploic artery) for long distances[12,45-48]. Anastomoses to be made with a microscope will increase the success. HA reconstruction can be beneficial in early postoperative injuries since liver necrosis occurs within the first 4 d. Li et al[12] reported that liver ischemia could be resolved before liver necrosis and atrophy develop in patients who undergo reconstruction within 4 d. However, reconstruction may not always be possible.
In planned ligations and embolizations, there is no need for additional procedures. In cases where artery resection is performed due to tumor invasion, there are different approaches regarding whether reconstruction is performed or not[12,15,20,45,47,48]. Primary reconstruction should be performed in planned resections due to benign pathologies (pseudoaneurysm, aneurysm, etc.). There is no need for additional procedures and clinical follow-up of the patient in planned ligations and embolizations. All patients with or without reconstruction should be closely monitored for liver ischemia and early diagnosis and management of potential complications (Figure 4).
In HA injuries caused by penetrating or blunt trauma, the patient's hemodynamic status and the presence of acute abdominal findings (perforation) determine the treatment approach to be performed. Embolization is the first procedure to be performed in hemodynamically stable patients who develop HA injury during perforating injuries or blunt traumas and invasive procedures[35]. It has also been reported that blood flow can be achieved, and bleeding can be controlled with an endovascular stent in selected cases[14,48]. In the series of 32 patients by Tzeng et al[14], they reported that initial hemostasis was achieved in 30 of the patients due to embolization applied to patients referred with liver trauma and HA injury. Cholecystitis development after embolization is rare and varies according to the location of the trauma. Cholecystitis is also seen very rarely in selective embolizations. The conservative approach is primarily recommended in cases with cholecystitis. Cholecystectomy is recommended in patients with gallbladder necrosis and emphysematous cholecystitis[42,49].
Problem-oriented approaches should be preferred in the management of complications. Antibiotics and percutaneous drainage procedures are recommended in cases with liver abscesses[41]. In liver necrosis cases and subsequent hepatic failure, early prostaglandin E1 administration, hemodiafiltration, and plasma exchange can help recover the liver[50]. However, liver transplantation remains the only option in patients with extensive necrosis and liver failure[3,51].
Primary reconstruction is recommended first for the injuries of rRHA, but there is no consensus on this issue[19,52]. There are many series that are not reconstructed in rRHA injuries or after resections (Table 2). Okada et al[52] think differently about rRHA resections. They reported that trying to protect the rRHA reduces the chances of R0 resection and concluded that resection should be performed when the tumor is adjacent to or very close to rRHA.
| Ref. | Cases, n | Etiology | Anomaly | HA injuries (laceration/transection, ligation/embolization) | Treatment options ligation/embolization reconstruction | Morbidity, n | Mortality, 90-d |
| Edgecombe and Gardner[2] | 40 | Review of the literature including cholecystectomy aneurysm and EHBT tumor | NA | 40 ligation | Ligation? Drainage | LF? LA? | 50%-75% |
| Brittain et al[16] | 5 | Cholecystectomy | NA | 5 injury/ligation | No reconstruction, drainage | 1% LA | 1% |
| Alves et al[15] | 55 | Postcholecystectomy biliary tract stricture series (Bismuth type 3-4-5) | 20? | 20 rRHA/RHA injury?, 2 HA pseudoaneurism, 4 portal vein injury | 43 HJ, 12 right Hx | 1% LA, 12% atrophy | NA |
| Stewart et al[10] | 261 | Biliary tract injury during LC | NA | 84 RHA injury | 4 Hx, HJ, drainage | 12% LA, 9% LN, 17% bleeding, 7% hemobilia, … | 2% |
| Gaujoux et al[24] | 545 | PD series | NA | 4 injury (Postoperative detection) | 4 (Thrombectomy, stenting, reconstruction) 2 left Hx | 2% LN, 4% thrombosis, 1% HJ | 3% |
| Tzeng et al[14] | 32 | Liver trauma (15) + Interventional HA injury (17) | NA | 32 injury | 32 Embolization (2 fail) | 2% LA, drainage | - |
| Li et al[12] | 60 | Biliary tract injury during LC | NA | 8 RHA injury; 2 PHA injury | 5 Reconstruction (2 fail) | 3 LN, 3 Hx, 2 LA, 3 others | 3% |
| Turrini et al[53] | 471 | PD series | 47 | 1 injury? 2 planned resection | 2 Reconstruction | - | - |
| Eshuis et al[9] | 758 | PD series | 143 | 8 planned resection, 5 injury | 3 Reconstruction | 3 PF, 4 DGE, 1 LA, 3 Rlp | 2% |
| Okada et al[52] | 180 | PD series | 25 | 6 preop embolization and planned resection | No-reconstruction | 1 POPF | - |
| El Amrani et al[27] | 2278 | Systematic analysis for PD (1950-2014) | 440 | 49 injury; 6 embolization (preop) | 18 Reconstruction | POPF 15%, DGE 39%, | 0%-10% |
| Landen et al[3] | NA | Systematic review for PD (1990-2016) | 3 | 21 injury (8 PHA, 3 RHA, 3 rRHA, 4 HA thrombosis, 3 HA injury) | 5 Reconstruction (1 fail) | 14 LA, 3 LF, 6 AL,11 Rlp | 5 (24%) |
| Asano et al[19] | 343 | PD series | 51 | 1 rRHA injury; 8 rRHA planned resection | No reconstruction; 1 drainage | 1 LA | - |
| Kleive et al[20] | 1535 | Pancreatectomy series | NA | 14 injury (5 SMA, 5 RHA, 2 CHA, 2 Celiac trunk); 22 planned resection | Embolectomy, Hx, re-reconstruction, drainage | 4 thrombosis, 2 PPH, 1 POPF, 5 LN, 11 Rlp | 2 injured; 1 planned |
| Elsanousi et al[34] | 19 | Invasive HCC series | NA | 19 HALED | 19 HALED1 | 8 Ascites-controlled, 2 jaundice | 1 Pulmonary embolism |
Reconstruction could not be performed considering the resection area's width, the possibility of local recurrence, the occlusion of the arteries due to the tumor invasion process, and the liver's collateral compensation system.
Some various applications and procedures can be made to protect and increase liver blood supply and oxygenation. In the preoperative period, revealing HA and SMA's anatomy by radiological imaging plays a key role in preventing injury, preventing unnecessary procedures, and confirming the indication. Turrini et al[53] reported a preoperative radiological evaluation in which most of the radiologists reported that they saw the HA anomaly but did not reflect it in their reports. For this purpose, a detailed description of vascular formations in magnetic resonance angiography and computed tomography angiography will be instructive. Doppler ultrasonography may also contribute to the evaluation of arterial flow. It is even stated that deaths can be reduced by detecting embolism, thrombus, and stenosis (median arcuate ligament syndrome) with angiography performed in selected cases after surgery. Compliance with the radiological evaluation guidelines recommended by The Society of Abdominal Radiology and the American Pancreatic Association can minimize postoperative liver blood supply disorders[20,24,25].
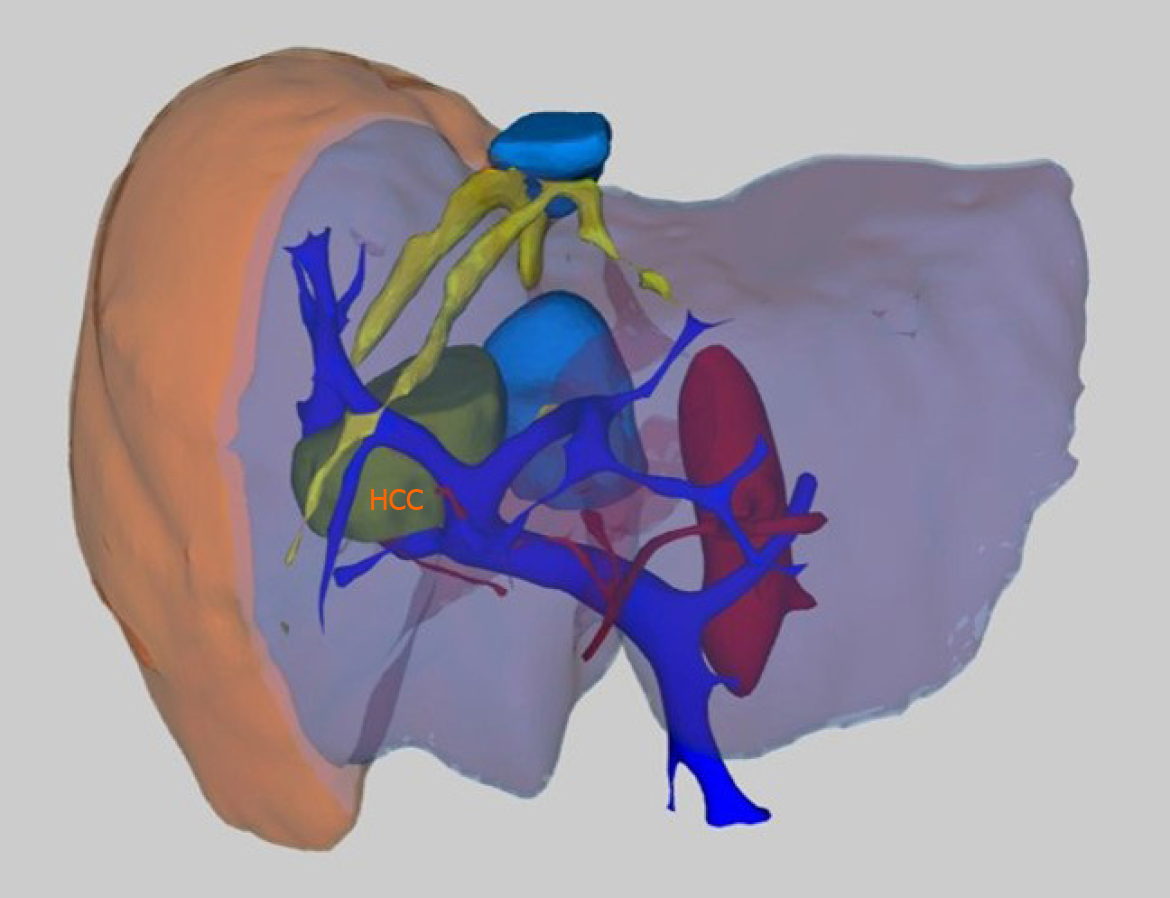
In recent years, studies on the definition of vascular structures in three dimensions using three-dimensional (3D) imaging and 3D printing technology have been started[54-56]. Detailed information about lesions, vascular anatomy, anomalies, the relationship with the lesion, and anatomical structures can be obtained, especially with the simulation studies to be performed in the preoperative period. In addition to revealing the pathologies and vascular anatomy with great accuracy, 3D imaging methods are also used to calculate the remnant liver volume (Figure 5). Training of residents with 3D imaging and 3D printing products and simulation applications for preoperative evaluation guide in determining the path to be followed in interventional procedures and surgeries. There are studies on the application of more radical and more protective procedures during surgery by obtaining specific models specific to patients and adapting them to the navigation systems to be applied[55,57].
It is vital to meet the oxygen demand of the liver. Twenty-five percent of the cardiac output (1250-1500 cc/min) goes to the liver. While 20%-25% of the blood coming to the liver is supplied by HA, 75%-80% of it is supplied by the portal vein. Forty percent to 50% of oxygen is provided by HA, and the portal vein provides 50%-60%. While the portal vein blood's oxygen saturation is 50%-60%, the HA oxygen saturation is over 90%[3,58]. In case of interruption of HA flow, the deficiency is compensated by portal vein flow. In a patient with impaired hemodynamics, the portal vein's oxygen parameters deteriorate further, and the liver's oxygenation is disrupted. The presence of hypovolemia, dehydration, anemia, lung problems, pain, excessive sedation, limitation of movement, or heart problems will further increase the risks associated with artery ligation[44,58]. Struggle with shock and providing oxygenation are the first protective and therapeutic procedures.
Exposing the HA and SMA and controlling HA pulses by closing the flow before cutting the gastroduodenal artery are among the first procedures to be performed. The most important reasons for the development of HA trauma are careless dissection and inadvertent transection. The posterior approach in surgery (arteria is first) can prevent the rRHA injury[59]. The development of portal vein injury with HA is frequently mortal[3,60].
The extent of liver tissue damage that will occur as a result of HA trauma in patients with obstructive jaundice is greater. This is due to the high bilirubin levels and increased bile acids in the blood, further aggravating ischemia in liver cells. The presence of sepsis also aggravates this situation. HA blood flow plays a vital role in the clearance of bacteria from the portal vein to the liver. Necrosis resulting from the cessation of current also facilitates bacterial colonization. In experimental studies, it has been shown that animals whose HA was ligated and given antibiotics had a higher survival chance[16].
Portal blood flow and collaterals also gain critical importance in cases of HA injury. Oxygenation of the portal vein and collaterals will play an essential role in preventing the development of necrosis and survival[16,21,61]. It has been demonstrated that there are 26 different collateral pathways around the liver[21]. The presence of collaterals can be demonstrated with enhanced computed tomography. The inferior phrenic artery, superior falciform ligament artery, right triangular ligament artery, and omental and subcapsular collaterals can significantly contribute to preventing necrosis[62]. Yoshida et al[63] found dense collaterals (communications) between the capsular arterial plexus and intrahepatic isolated hepatic arteries. There is also a blood supply between the right and left hepatic arteries through the hilar plate plexus. Hilar plate plexus also provides blood supply of the collateral network around the common bile duct confluence and contributes to healing the hepaticojejunostomy anastomosis[64]. In cases where the artery revision is not successful, it should be tried to make the hepaticojejunostomy anastomosis close to the hilar plate, considering that the blood supply may be better[12]. In cases where artery reconstruction cannot be performed, (subhepatic) drainage is also recommended[16].
Arterialization of the portal vein was applied as a salvage procedure to increase portal blood oxygenation in HA thrombosis after liver transplantation. It has been applied from time to time in borderline pancreatic tumors and selected cases. It can be applied when HA repair is not possible or after resections[65,66]. The anastomosis can be performed in many places included in the portal system. The anastomosis can be made with autologous or synthetic (Gore-Tex, polytetrafluoroethylene graft) grafts or between arteries and veins (e.g., iliac artery-middle colic vein / superior mesenteric vein, colic artery branches, and ileocolic vein, etc.). It should be preferred to do it with a microscope/loop. It can also be done using the transected HA stump. In cases where the arterial flow rate is high, portal hypertension clinics may develop. In such cases, the anastomosis may need to be closed by interventional methods (embolization, coiling)[65,66].
Control and follow-up of transaminases in the early postoperative period can provide important clues. In the case of high transaminases (> 2000 U/L), additional radiological imaging methods are recommended to investigate the extent of the ischemic condition and to perform reconstruction in cases with HA trauma[67]. Serum transaminases controlled serially in the early period in the follow-up of patients with or suspected HA trauma might be instructive about the extent of the damage and prognosis. In the 2894 PD series conducted in 25 years by John Hopkins, it was emphasized that there might be a serious relationship between the increase in serum transaminases and clinical progression and prognosis. In this study, it has been shown that if the serum transaminases peak level rises from < 500 U/L to 2000 U/L and above, the mortality may increase from 0.9% to 29%[67]. They also reported that almost all patients could recover if transaminases remained below 1000 U/L. In the same series, mortality was reported to be 7%, 3%, and 0.9% in patients with low albumin (< 2.5 g/dL), medium (2.6-3.5 g/dL), and high (> 3.5 g/dL), respectively.
It has been reported in experienced centers that HA trauma will be extremely rare. In the PD series of 434 cases by Kulkarni et al[47], they reported only 2 HA trauma. In the PD series of 1535 cases published in Oslo, Sweden, it was reported that only 8 patients (0.52%) had HA trauma[20].
Since HA trauma may develop in some of the patients who develop bile duct trauma[12,13,10], it is more appropriate to perform the intervention in experienced centers for patients who are planned to undergo reoperation for revision purposes.
The weaknesses of our study are the scarcity of clinical series in the literature and the fact that there are studies mostly in the form of case reports. Another point is that prospective studies on HA trauma are limited to experimental and subjects only, and these studies cannot be performed in humans. More comprehensive data can be obtained with the follow-up and evaluation of HA traumas encountered with multicentric study protocols.
Complications and deaths due to HA trauma are less common today. Repair should be attempted in all cases where HA trauma is detected (during surgery and early postoperative period). Arterial flow can be maintained with primary anastomosis, arterial transpositions, or grafts.
Liver failure, liver abscess, anastomotic opening, and bile duct stricture are the most common complications. The risk of complications increases in patients with hemodynamic instability, jaundice, cholangitis, and sepsis. The cause of death is often liver necrosis, sepsis, and liver failure. Antibiotic use and drainage reduce the risks.
To be protected from HA trauma, performing adequate radiological evaluation before the operation, revealing the variations, and determining the appropriate approach plan will minimize the risks.
HA trauma is a much less common complication, especially in HPB surgery centers. Considering the possibility of accompanying HA trauma in cases where bile duct trauma develops, the patient should be directed to HPB surgery centers if possible.
Large-scale and multicentric studies are needed to understand better the early and long-term consequences of HA trauma and develop preventive procedures.
The hepatic artery (HA) has been used more and more for diagnosis and treatment in recent years. Besides, HA is one of the most threatened vascular structures during hepatopancreatobiliary (HPB) surgeries and interventional procedures. The incidence of HA injury is not precisely known.
There are many studies reporting that more than half of the cases died in the case of HA trauma or involuntary ligation until the last 3-4 decades. There is still a risk of serious morbidity and mortality as a result of injury to the HA during an increasing number of interventional procedures and HPB surgeries in recent years. There is a need for algorithmic approaches to HA-related problems and their solutions, which can be encountered for many different reasons.
Most of the studies related to HA in the literature are in the form of case reports. There are no algorithms developed for solving HA problems that surgeons, internists, gastroenterologists, hepatologists, and interventional radiologists often encounter sporadically. There is no consensus established for the solution of the problems encountered. Since there are no experimental studies in humans, there is a need for the analysis of data from case reports and a limited number of clinical series.
The authors have reviewed 100 years of MEDLINE (PubMed) literature. The clinical approach algorithm was tried to define by reviewing the papers, including cases and series of HA injuries. The study researched keywords containing "hepatic artery" AND (trauma OR injury OR resection OR ligation OR avulsion OR transection OR reconstruction) and their various combinations. Approaches that have evolved in recent years in managing patients with HA injury (laceration, transection, ligation, resection) with severe morbidity and mortality risk are reviewed in the light of current literature.
The authors found 6314 articles as a result of the MEDLINE research. While one to two articles were published annually in the first 50 years, this number has increased gradually in the last 50 years and reached 109-237 articles per year. There are 1555 articles with the keywords "hepatic artery injury" or "hepatic artery trauma" and 468 articles with the word "hepatic artery ligation". In the first half of the century, we detected that HA traumas were applied mainly with unintentional ligations during gallbladder and stomach surgeries and patients who underwent planned ligation for HA aneurysm. We have identified 57 studies with the word "HA resection", and "HA embolization" was the subject of 406 studies; most of them have been published in the previous 2 decades. Articles on HA transection and reconstruction, a challenging stage of liver transplantation, ligation, resection, and reconstructions for HA aneurysms were also discussed. HA embolization has found many more clinical applications with the developing technological applications, and more studies have been reported in recent years. Here, HA pathologies, therapeutic procedures, and also HA embolization will be shortly described in the paper.
With the technological developments in the last 2-3 decades and their contribution to diagnosis and treatment, positive developments have been identified in the prevention and management of HA trauma and related complications. The risk of HA injury increases during cholecystectomies and pancreatoduodenectomies, among the most common operations. HA anatomy shows anomalies in approximately 15%-25% of the cases, further increasing this risk. Complications and deaths due to HA injury are less common today. The risk of complications increases in patients with hemodynamic instability, jaundice, and cholangitis. Revealing the variations in the preoperative radiological evaluation will reduce the risks. In cases where HA injury is detected, arterial flow continuity should be tried to maintain with primary anastomosis, arterial transpositions, or grafts. In cases where bile duct injury develops, patients should be directed to HPB surgery centers, considering the possibility of accompanying HA injury.
Due to the high risks it contains, the inability to conduct prospective studies in humans remains a problem. However, experimental studies in animals are needed regarding identified pathological processes. Besides, large-scale and multicentric clinical prospective studies are needed to understand better the early and long-term results of HA ligation and determine preventive procedures.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Urade T S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | Graham RR, Cannell D. Accidental ligation of the hepatic artery. Report of one case, with a review of the cases in the literature. Br J Surg. 1933;20:566-579. [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Edgecombe PW, Gardner C. Accidental ligation of the hepatic artery and its treatment. Can Med Assoc J. 1951;64:518-522. [PubMed] [Cited in This Article: ] |
| 3. | Landen S, Ursaru D, Delugeau V, Landen C. How to deal with hepatic artery injury during pancreaticoduodenectomy. A systematic review. J Visc Surg. 2017;154:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Haberer JP, Audibert G, Saunier CG, Muller C, Laxenaire MC, Hartemann D. Effect of propofol and thiopentone on regional blood flow in brain and peripheral tissues during normoxia and hypoxia in the dog. Clin Physiol. 1993;13:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Huggins C, Post J. Experimental subtotal ligation of the arteries supplying the liver. Arch Surg. 1937;35:878. [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Markowitz J, Rappaport A, Scott AC. Prevention of liver necrosis following ligation of hepatic artery. Proc Soc Exp Biol Med. 1949;70:305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Tanturi C, Swigart LL, Canepa JF. Prevention of death from experimental ligation of the liver (hepatic proper) branches of the hepatic artery. Surg Gynecol Obstet. 1950;91:680-704. [PubMed] [Cited in This Article: ] |
| 8. | Müller P, Randhawa K, Roberts KJ. Preoperative identification of anomalous arterial anatomy at pancreaticoduodenectomy. Ann R Coll Surg Engl. 2014;96:e34-e36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Eshuis WJ, Olde Loohuis KM, Busch OR, van Gulik TM, Gouma DJ. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB (Oxford). 2011;13:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Stewart L, Robinson TN, Lee CM, Liu K, Whang K, Way LW. Right hepatic artery injury associated with laparoscopic bile duct injury: incidence, mechanism, and consequences. J Gastrointest Surg. 2004;8:523-530; discussion 530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Gordon-Taylor G. Rare Cause of Severe Gastro-intestinal Haemorrhage. Br Med J. 1943;1:504-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Li J, Frilling A, Nadalin S, Paul A, Malagò M, Broelsch CE. Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br J Surg. 2008;95:460-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 911] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 14. | Tzeng WS, Wu RH, Chang JM, Lin CY, Koay LB, Uen YH, Tian YF, Fong Y. Transcatheter arterial embolization for hemorrhage caused by injury of the hepatic artery. J Gastroenterol Hepatol. 2005;20:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Alves A, Farges O, Nicolet J, Watrin T, Sauvanet A, Belghiti J. Incidence and consequence of a hepatic artery injury in patients with postcholecystectomy bile duct strictures. Ann Surg. 2003;238:93-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Brittain RS, Marchioro TL, Hermann G, Waddell WR, Starzl TE. Accidental Hepatic Artery Ligation in Humans. Am J Surg. 1964;107:822-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Daseler EH, Anson BJ. The cystic artery and constituents of the hepatic pedicle; a study of 500 specimens. Surg Gynecol Obstet. 1947;85:47-63. [PubMed] [Cited in This Article: ] |
| 18. | Kim KM, Park JW, Lee JK, Lee KH, Lee KT, Shim SG. A Comparison of Preoperative Biliary Drainage Methods for Perihilar Cholangiocarcinoma: Endoscopic vs Percutaneous Transhepatic Biliary Drainage. Gut Liver. 2015;9:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Asano T, Nakamura T, Noji T, Okamura K, Tsuchikawa T, Nakanishi Y, Tanaka K, Murakami S, Ebihara Y, Kurashima Y, Shichinohe T, Hirano S. Outcome of concomitant resection of the replaced right hepatic artery in pancreaticoduodenectomy without reconstruction. Langenbecks Arch Surg. 2018;403:195-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kleive D, Sahakyan MA, Khan A, Fosby B, Line PD, Labori KJ. Incidence and management of arterial injuries during pancreatectomy. Langenbecks Arch Surg. 2018;403:341-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 516] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Mcfadzean AJ, Cook J. Ligation of the splenic and hepatic arteries in portal hypertension. Lancet. 1953;1:615-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Woolling KR, Baggenstoss AH, WEIR JF. Infarcts of the liver. Gastroenterology. 1951;17:479-493. [PubMed] [Cited in This Article: ] |
| 24. | Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Yang Y, Jiang N, Lu MQ, Xu C, Cai CJ, Li H, Yi SH, Wang GS, Zhang J, Zhang JF, Chen GH. [Anatomical variation of the donor hepatic arteries: analysis of 843 cases]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1164-1166. [PubMed] [Cited in This Article: ] |
| 26. | Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | El Amrani M, Pruvot FR, Truant S. Management of the right hepatic artery in pancreaticoduodenectomy: a systematic review. J Gastrointest Oncol. 2016;7:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 28. | Rubio-Manzanares-Dorado M, Marín-Gómez LM, Aparicio-Sánchez D, Suárez-Artacho G, Bellido C, Álamo JM, Serrano-Díaz-Canedo J, Padillo-Ruiz FJ, Gómez-Bravo MÁ. Implication of the presence of a variant hepatic artery during the Whipple procedure. Rev Esp Enferm Dig. 2015;107:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Oki E, Sakaguchi Y, Hiroshige S, Kusumoto T, Kakeji Y, Maehara Y. Preservation of an aberrant hepatic artery arising from the left gastric artery during laparoscopic gastrectomy for gastric cancer. J Am Coll Surg. 2011;212:e25-e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Cirocchi R, D'Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, Gioia S, Lancia M, Artico M, Randolph J. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surgeon. 2020;18:100-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 31. | Ferrada P, Ratnasekera A, Khokar A. Penetrating Traumatic Laceration of Common Hepatic Artery and Portal Vein: A Rare Story of Success. Am Surg. 2017;83:e148-e150. [PubMed] [Cited in This Article: ] |
| 32. | Petrelli NJ, Mittelman A. Hepatic artery ligation for liver cancer. In: Bottino JC, Opfell RW, Muggia FM, editors. Liver Cancer, Nijhoff Publishing, Boston, 1985: 143-156. [Cited in This Article: ] |
| 33. | Altemeier WA, McElhinney WT, Macmillan BG. Treatment of portal hypertension with hepatic artery ligation; an evaluation. AMA Arch Surg. 1955;71:571-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Elsanousi OM, Mohamed MA, Salim FH, Adam EA. Selective devascularization treatment for large hepatocellular carcinoma: Stage 2A IDEAL prospective case series. Int J Surg. 2019;68:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Ou J, Yu L, Nan-Sheng C. Ligation of the Left Hepatic Duct and Proper Hepatic Artery Following a Traffic Accident Injury. Indian J Surg. 2017;79:461-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Gachabayov M, Kubachev K, Mityushin S, Zarkua N. Recurrent Hemobilia Due to Right Hepatic Artery Pseudoaneurysm. Clin Med Res. 2017;15:96-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Bacalbasa N, Brezean I, Anghel C, Barbu I, Pautov M, Balescu I, Brasoveanu V. Successful Resection and Vascular Ligation of a Large Hepatic Artery Aneurysm - A Case Report and Literature Review. In Vivo. 2017;31:979-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Bradke D, Tran A, Ambarus T, Nazir M, Markowski M, Juusela A. Grade III subcapsular liver hematoma secondary to HELLP syndrome: A case report of conservative management. Case Rep Womens Health. 2020;25:e00169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Chen HM, Fu ZH, Deng DF, Huang JZ, Zhang X, Xu ZQ, Wang YD. [The safety and efficacy of combined hepatic artery resection in treatment of hilar cholangiocarcinoma: a meta-analysis]. Zhonghua Yi Xue Za Zhi. 2021;101:286-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Peters NA, Javed AA, Cameron JL, Makary MA, Hirose K, Pawlik TM, He J, Wolfgang CL, Weiss MJ. Modified Appleby Procedure for Pancreatic Adenocarcinoma: Does Improved Neoadjuvant Therapy Warrant Such an Aggressive Approach? Ann Surg Oncol. 2016;23:3757-3764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Hasegawa K, Kubota K, Aoki T, Hirai I, Miyazawa M, Ohtomo K, Makuuchi M. Ischemic cholangitis caused by transcatheter hepatic arterial chemoembolization 10 mo after resection of the extrahepatic bile duct. Cardiovasc Intervent Radiol. 2000;23:304-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T, Hayashi K, Matsunaga N. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Jadrijevic S, Sef D, Kocman B, Mrzljak A, Matasic H, Skegro D. Right hepatectomy due to portal vein thrombosis in vasculobiliary injury following laparoscopic cholecystectomy: a case report. J Med Case Rep. 2014;8:412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Kishi Y, Kajiwara S, Seta S, Hoshi S, Hasegawa S, Hayashi Y, Sasaki K. Cholangiojejunal fistula caused by bile duct stricture after intraoperative injury to the common hepatic artery. J Hepatobiliary Pancreat Surg. 2002;9:125-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Halasz NA. Cholecystectomy and hepatic artery injuries. Arch Surg. 1991;126:137-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Bozdağ AD, Peker Y, Ozer M, Derici H, Uluç E. Vascular reconstruction of hepatic artery injury using the gastroduodenal artery: 6-year follow-up-case report. J Trauma. 2002;52:780-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 47. | Kulkarni GV, Malinowski M, Hershberger R, Aranha GV. Proper hepatic artery reconstruction with gastroduodenal artery transposition during pancreaticoduodenectomy. Perspect Vasc Surg Endovasc Ther. 2013;25:69-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Faulds J, Johner A, Klass D, Buczkowski A, Scudamore CH. Hepatic artery transection reconstructed with splenic artery transposition graft. Perspect Vasc Surg Endovasc Ther. 2012;24:87-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Allison DJ, Jordan H, Hennessy O. Therapeutic embolization of the hepatic artery: a review of 75 procedures. Lancet. 1985;1:595-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, Kato K, Hayano K, Kadowaki S, Shibuya M, Maeno S, Takada T, Eguchi T. Eleven cases of postoperative hepatic infarction following pancreato-biliary surgery. J Gastrointest Surg. 2010;14:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Ringe B, Pichlmayr R. Total hepatectomy and liver transplantation: a life-saving procedure in patients with severe hepatic trauma. Br J Surg. 1995;82:837-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Okada K, Kawai M, Hirono S, Miyazawa M, Shimizu A, Kitahata Y, Tani M, Yamaue H. A replaced right hepatic artery adjacent to pancreatic carcinoma should be divided to obtain R0 resection in pancreaticoduodenectomy. Langenbecks Arch Surg. 2015;400:57-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Turrini O, Wiebke EA, Delpero JR, Viret F, Lillemoe KD, Schmidt CM. Preservation of replaced or accessory right hepatic artery during pancreaticoduodenectomy for adenocarcinoma: impact on margin status and survival. J Gastrointest Surg. 2010;14:1813-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Moglia A, Di Franco G, Morelli L. Use of 3D models for planning, simulation, and training in vascular surgery. Updates Surg. 2019;71:185-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Perica ER, Sun Z. A Systematic Review of Three-Dimensional Printing in Liver Disease. J Digit Imaging. 2018;31:692-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Zeng N, Yang J, Xiang N, Wen S, Zeng S, Qi S, Zhu W, Hu H, Fang C. [Application of 3D visualization and 3D printing in individualized precision surgery for Bismuth-Corlette type Ⅲ and Ⅳ hilar cholangiocarcinoma]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:1172-1177. [PubMed] [Cited in This Article: ] |
| 57. | Fang C, Fang Z, Fan Y, Li J, Xiang F, Tao H. [Application of 3D visualization, 3D printing and 3D laparoscopy in the diagnosis and surgical treatment of hepatic tumors]. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:639-645. [PubMed] [Cited in This Article: ] |
| 58. | Le Roy B, Dupré A, Gallon A, Chabrot P, Gagnière J, Buc E. Liver hypertrophy: Underlying mechanisms and promoting procedures before major hepatectomy. J Visc Surg. 2018;155:393-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Jiang X, Yu Z, Ma Z, Deng H, Ren W, Shi W, Jiao Z. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int J Surg. 2020;73:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Hackert T, Stampfl U, Schulz H, Strobel O, Büchler MW, Werner J. Clinical significance of liver ischaemia after pancreatic resection. Br J Surg. 2011;98:1760-1765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Jefferson NC, Hassan MI, Popper HL, Necheles H. Formation of effective collateral circulation following excision of hepatic artery. Am J Physiol. 1956;184:589-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Ibukuro K, Mori M, Akita K. The hepatic capsular arteries: imaging features and clinical significance. Abdom Radiol (NY). 2019;44:2729-2739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Yoshida K, Matsui O, Miyayama S, Ibukuro K, Yoneda N, Inoue D, Kozaka K, Minami T, Koda W, Gabata T. Isolated Arteries Originating from the Intrahepatic Arteries: Anatomy, Function, and Importance in Intervention. J Vasc Interv Radiol. 2018;29:531-537.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Vellar ID. The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust N Z J Surg. 1999;69:816-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Hokuto D, Nomi T, Yamato I, Yasuda S, Obara S, Yamada T, Kanehiro H, Nakajima Y. Hepatic artery injury during left hepatic trisectionectomy for colorectal liver metastasis treated by portal vein arterialization. Int J Surg Case Rep. 2015;13:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Bhangui P, Salloum C, Lim C, Andreani P, Ariche A, Adam R, Castaing D, Kerba T, Azoulay D. Portal vein arterialization: a salvage procedure for a totally de-arterialized liver. The Paul Brousse Hospital experience. HPB (Oxford). 2014;16:723-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Winter JM, Cameron JL, Yeo CJ, Alao B, Lillemoe KD, Campbell KA, Schulick RD. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007;204:1029-36; discussion 1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |