Published online Jul 16, 2021. doi: 10.12998/wjcc.v9.i20.5568
Peer-review started: December 11, 2020
First decision: April 4, 2021
Revised: April 14, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: July 16, 2021
Glycated hemoglobin (Hb) (HbA1c) is an indicator that is used to diagnose and monitor the treatment of diabetes. Many factors can affect the detection of HbA1c. One of the most important of these factors is the Hb variant. Here, we report a rare Hb variant and evaluate its effect on HbA1c.
A 35-year-old man was suspected of harboring an Hb variant following the measurement of HbA1c with the Variant II Turbo 2.0 Hb detection system during a routine examination. Subsequently, we used the Arkray HA-8160 and ARCHITECT c4000 system to reanalyze HbA1c. Finally, the Hb variant was detected with a Capillary2FP analyzer that operates on the principle of capillary electrophoresis. We also used gene sequencing to investigate the mutation site. The value of HbA1c detected with the Variant II Turbo 2.0 system was 52.7%. However, the Arkray HA-8160 system did not display a result while the ARCHITECT c16000 system showed a result of 5.4%. The Capillary2FP analyzer did not reveal any abnormal Hb zones. However, gene sequencing identified the presence of a mutation in the Hb β2 chain [CD2(CAC>TAC), His>Tyr, HBB: c.7C>T]; the genotype was Hb Fukuoka.
Hb variants could cause abnormal HbA1c results. For patients with Hb variants, different methods should be used to detect HbA1c.
Core Tip: Hemoglobin A1c (HbA1c) is an indicator of diabetes diagnosis and blood glucose monitoring. Therefore, the accuracy of HbA1c results is of great significance to clinical diagnosis and treatment. This case had an abnormal HbA1c result and traced back to a rare hemoglobin variant. Hemoglobin variants are one of the important factors affecting the accuracy of HbA1c results. In this case, different methods were used to detect HbA1c, which can provide reference evidence for subsequent cases and reduce false results reports.
- Citation: Lin XP, Yuan QR, Niu SQ, Jiang X, Wu ZK, Luo ZF. Hemoglobin Fukuoka caused unexpected hemoglobin A1c results: A case report. World J Clin Cases 2021; 9(20): 5568-5574
- URL: https://www.wjgnet.com/2307-8960/full/v9/i20/5568.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i20.5568
Hemoglobin A1c (HbA1c) is the main indicator recommended by the American Diabetes Association and the World Health Organization for the diagnosis of diabetes[1]. HbA1c is a stable compound that covalently bonds glucose and hemoglobin (Hb) β-chain N-terminal valine residues in human blood. It reflects the average blood glucose concentration over the previous 2 mo to 3 mo. However, the results of the HbA1c are affected by a wide range of factors, including Hb variants, severe hemolytic anemia, and severe liver disease, etc. The most common factors that interfere with HbA1c tests are Hb variants[2,3]. In this paper, we report a case with abnormal HbA1c values, which was eventually diagnosed as a rare Hb mutation.
A 35-year-old man arrived at our hospital for a physical examination in November 2018.
The patient had no special past history.
The patient had no special personal and family history.
Normal physical examination without any obvious signs of abnormality.
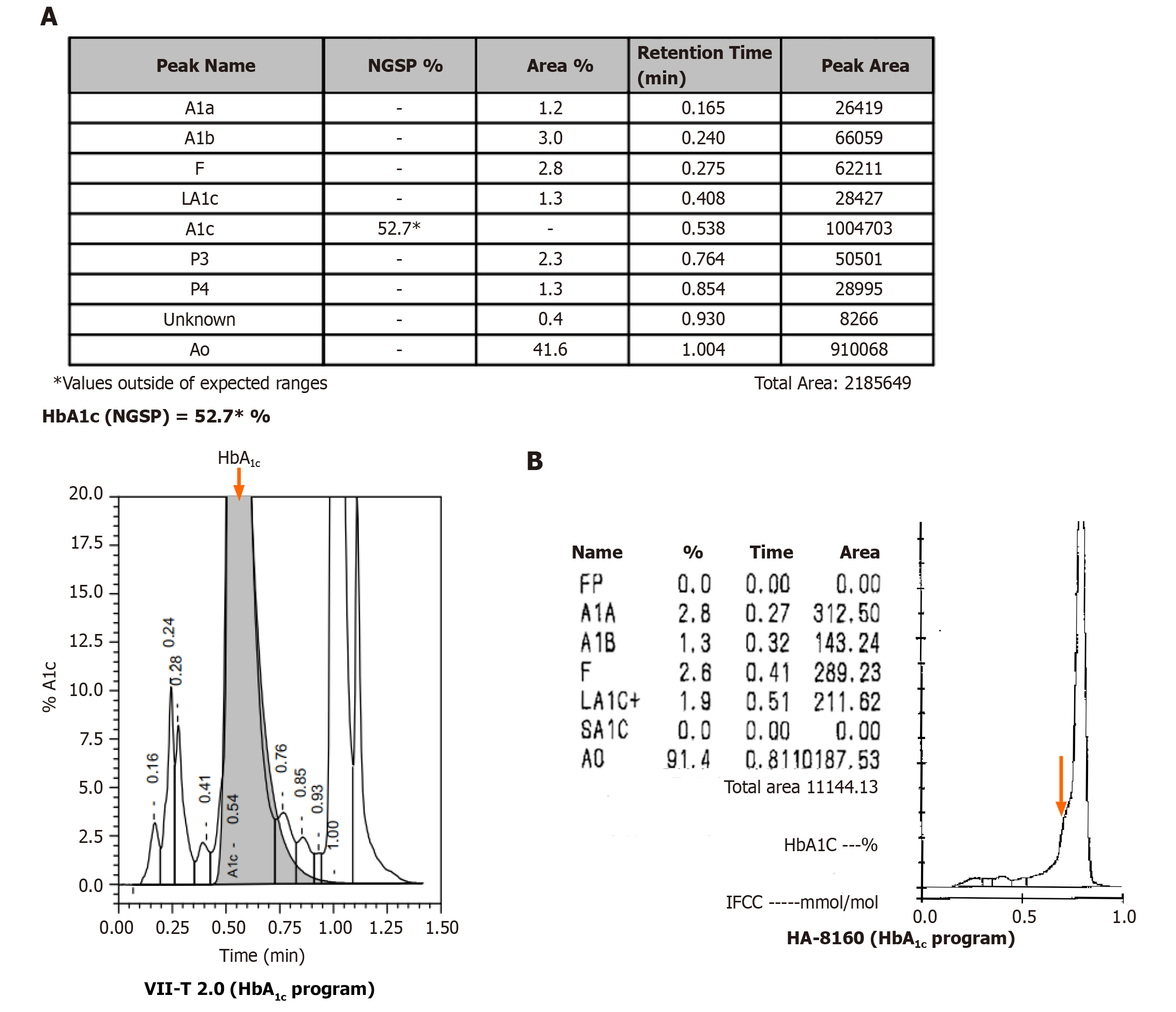
The HbA1c result of 52.7% was out of the expected detection range (Figure 1A). The HbA1c of the patient was remarkably elevated in comparison to the reference range (4.0%-6.4%). Data arising from routine blood analyses are shown in Table 1. The patient had normal liver function, normal fasting glucose, and no previous history of diabetes. The HbA1c test was performed using the Biorad Variant II Turbo 2.0 automatic glycated Hb analyzer (Biorad, United States); the HbA1c value was abnormally elevated, although the patient had no history of diabetes. Therefore, we suspected that the patient possessed Hb variants that interfered with the results of the HbA1c test.
| Results | Reference range | |
| Red blood cells (× 1012/L) | 5.6 | 4.3-5.8 |
| Hemoglobin (g/L) | 161 | 130-175 |
| Mean erythrocyte volume (fL) | 91 | 82-100 |
| Mean erythrocyte hemoglobin volume (pg) | 29 | 27-34 |
| Mean erythrocyte hemoglobin concen-tration (g/L) | 315 | 316-354 |
| Erythrocyte distribution width (%) | 13 | 12-15 |
| Total protein (g/L) | 79.3 | 65.0-85.0 |
| Albumin (g/L) | 46.1 | 32.0-45.0 |
| Alanine aminotransferase (U/L) | 19 | 0-55 |
| Aspartate transferase (U/L) | 22 | 15-40 |
| Blood creatinine (µmol/L) | 76 | 64-104 |
| Blood urea nitrogen (mmol/L) | 4.8 | 3.2-7.4 |
| Fasting glucose (mmol/L) | 4.71 | 3.90-6.10 |
We attempted to confirm the HbA1c value by using the Arkray HA-8160 automatic glycated Hb analyzer (Arcolai, Japan), which features high-performance liquid chromatography (HPLC), and the ARCHITECT c4000 system (Abbott, United States), which operates by enzymatic methods. The Arkray HA-8160 system returned no result; the instrument showed that the HbA1c peak did not appear, and there was an abnormal protruding peak on the left of peak A0 (Figure 1B). The ARCHITECT c16000 enzyme assay returned a result of 5.4%.
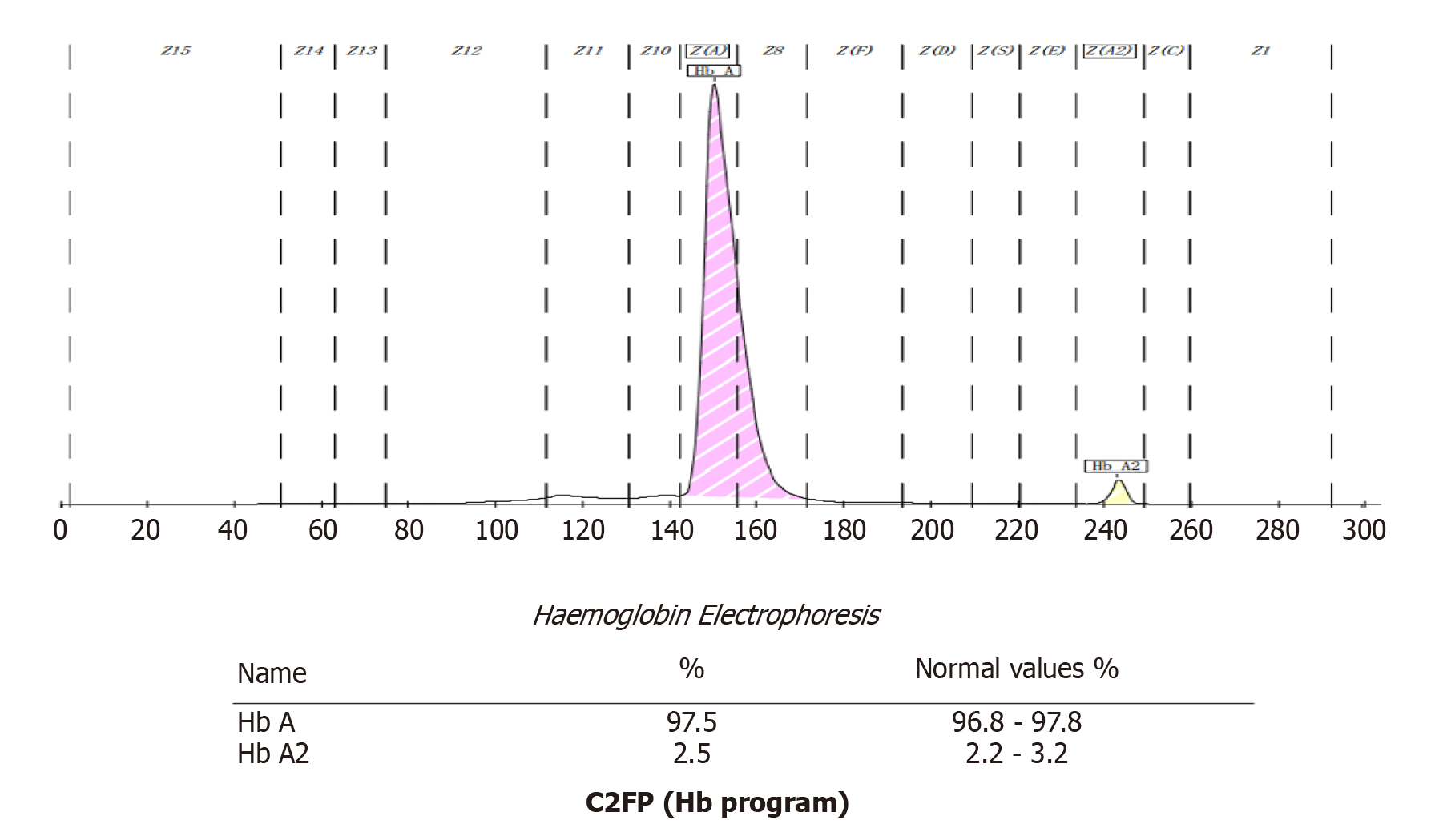
Next, we used the Capillary2FP automated Hb analyzer (Sebia, France) to find the presence of abnormal Hb. Capillary electrophoresis showed no abnormal Hb bands (Figure 2).
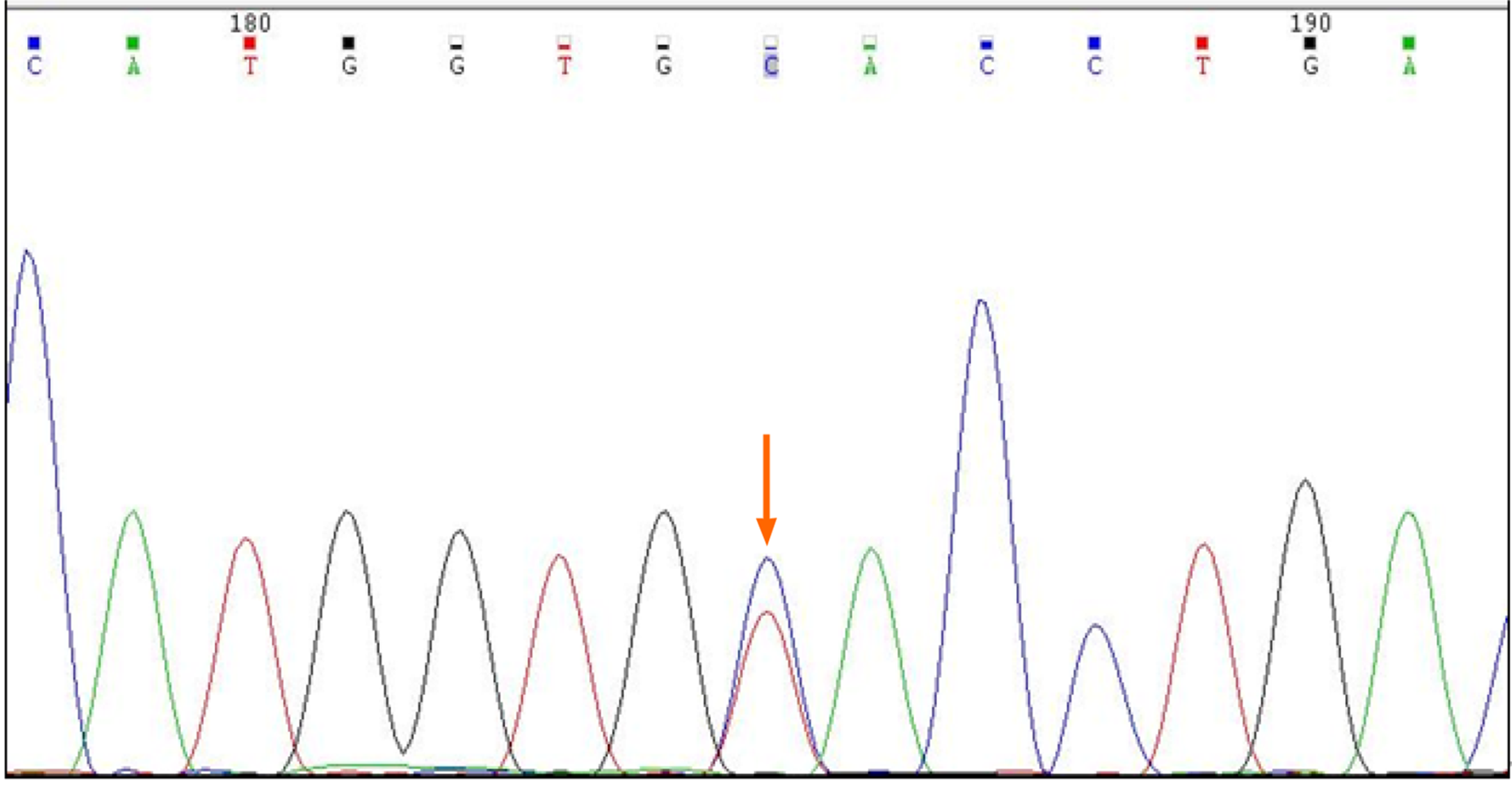
In addition, the patient's genomic DNA was extracted using a Genomic DNA Isolation Kit (Kaipu, China) and the HBA1, HBA2, and HBB genes were amplified with specific forward/reverse primers and sequenced on an ABI3500 sequencer. HBA1 forward primer was 5´-CGCGCCAGCCAATGAGC-3´ and HBA1 reverse primer was 5´-ACACACA TGGCTAGAACCTCTCTG-3´. HBA2 forward primer was 5´-GGGCT CCGCGCCAGCCAA-3´ and HBA2 reverse primer was 5´-CAAGGACCTCTCT GCAGCT-3´. HBB forward primer was 5´-TACGGCTGTCATCACTTAG-3´and HBB reverse primer was 5´-GCCACACTGAGTGAGCTGCACT-3´. We extracted a sample of whole blood from the patient and extracted genomic DNA. This was used as a template to amplify the Hb gene. Polymerase chain reaction amplicons were then sequenced. No thalassemia mutation types were found in the sequencing range for α1 and α2. However, the β-globin gene possessed a histidine > tyrosine (CAC >TAC) mutation in the 7th amino acid, C>T (Figure 3). Next, we searched the HbA1c variant library website (http://globin.bx.psu.edu/hbvar/menu.html), which revealed that the Hb genotype was Hb Fukuoka (HBB:c.7C>T). Finally, the patient was confirmed to possess a heterozygous Hb Fukuoka mutation.
Finally, the patient was confirmed to possess a heterozygous Hb Fukuoka mutation.
The patient was not treated.
For patients with Hb variants, we recommend the use of an assay that is not affected by interference caused by Hb variants.
The guidelines relating to diabetes mellitus that were published by the American Chemical Society in 2011 suggest that a patient should receive further research/investigation if HbA1c > 15% or if the laboratory data are inconsistent with clinical manifestations. The guidelines also recommend the use of different test principles and methods so that the patient can be re-tested[4]. In this case, the patient’s HbA1c was 52.7%, significantly higher than 15%. The patient had no history of diabetes. Laboratory data were all within the normal quantitative range, including Hb, kidney and liver function parameters, and fasting blood glucose. Therefore, it was necessary to retest this patient and identify reasons that might underlie the abnormal HbA1c result.
Biorad Variant II Turbo 2.0 and Arkray HA-8160 Glycation Hemoglobin analyzers are based on HPLC technology. Using this technology, it is possible to separate Hb fractions by differential affinity to the internal surface of the column or to the buffer. The greater the affinity to the buffer, the faster the elution, and the shorter the retention time. The greater the affinity to the analytical column, the slower the elution, and the longer the retention time. In this case, the Biorad Variant II Turbo 2.0 returned an HbA1c result of 52.7%, and no result was detected by Arkray HA-8160. Therefore, we considered the possibility of interference caused by Hb variants. Hb variants can affect the separation of variants from HbA1c or HbA by changing their charge, co-eluting with HbA1c, and replacing glycosylation sites[5,6]. The NGSP website has declared that the common variants including S, C, D, and E have no effect on Biorad Variant II Turbo 2.0 (http://ngsp.org/factors.asp). However, some rare Hb variants can affect HbA1c with HPLC[7,8]. The ARCHITECT c16000 uses enzymatic methods to detect HbA1c values. By lysing the whole blood sample and performing extensive proteolytic digestion, the Hb β chain releases amino acids, especially the glycated N-terminal valine. The signal generated by the glycosylated valine in the subsequent color-based reaction can then be used to calculate the HbA1c. At the analytical level, these methods are unaffected by the presence of Hb variants, such as HbC, HbD, HbE, and HbS. However, this method is unable to detect Hb variants[9]. Therefore, an HbA1c result of 5.4% can accurately reflect the glycated Hb of the patient. The Capillary 2FP system operates on the principle of capillary electrophoresis and was used to investigate for the presence of an Hb variant. The Capillary 2FP system showed no abnormal Hb bands. Gene sequencing identified the variant as an Hb Fukuoka heterozygote. This variant was first reported in Japan in 1985, and subsequently reported by both Harano et al[10] and Farah et al[11]. Harano et al[10] found abnormal Hb peak in a diabetic patient's HbA1c test in 1990, and identified that the patient had Hb Fukuoka. Furthermore, Farah et al[11] identified the presence of Hb Fukuoka by performing electrophoresis examinations in a patient with anemia. As far as we know, this case was the first case to be discovered in China.
Several methods can be used to detect glycosylated Hb, including electrophoresis, immunoturbidimetric assay (immunoassay), enzymatic assay, boronate affinity, ion-exchange HPLC, or capillary electrophoresis. Laboratories should be aware of the limitations of their methods with respect to interference from other Hb variants and suggest alternative HbA1c quantification methods[12,13]. For patients with Hb variants, we recommend the use of an assay that is not affected by interference caused by Hb variants; this strategy will help avoid erroneous results that can mislead data in clinical practice[14]. Glycated albumin and fructosamine are recommended as alternative indicators to monitor glycaemia when HbA1c is disturbed[15].
The chromatographic pattern of abnormal Hb variants differs from that of normal samples, making it difficult for some instruments to determine accurate HbA1c values. HbA1c is an important indicator for the diagnosis, treatment, and monitoring of diabetes mellitus, and the accuracy of HbA1c results is of paramount importance. But meanwhile, it is important for laboratory staff to be familiar with the factors that may cause interference, and to be able to communicate with clinicians in a timely manner to clarify the situation and make clinical suggestions. The ultimate goal is to ensure accurate and realistic test results so as to help clinical diagnosis.
We are grateful for instructive discussion with Professor Luo ZF.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Panyasai S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Jia W. Standardising HbA1c-based diabetes diagnosis: opportunities and challenges. Expert Rev Mol Diagn. 2016;16:343-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. 2013;33:393-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol. 2019;72:12-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:e1-e47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 5. | Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3:446-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Nasir NM, Thevarajah M, Yean CY. Hemoglobin variants detected by hemoglobin A1c (HbA1c) analysis and the effects on HbA1c measurements. Int J Diabetes Dev Ctries. 2010;30:86-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Little RR, La'ulu SL, Hanson SE, Rohlfing CL, Schmidt RL. Effects of 49 Different Rare Hb Variants on HbA1c Measurement in Eight Methods. J Diabetes Sci Technol. 2015;9:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Strickland SW, Campbell ST, Little RR, Bruns DE, Bazydlo LAL. Recognition of rare hemoglobin variants by hemoglobin A1c measurement procedures. Clin Chim Acta. 2018;476:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Rhea JM, Molinaro R. Pathology consultation on HbA(1c) methods and interferences. Am J Clin Pathol. 2014;141:5-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Harano T, Harano K, Ueda S, Imai K, Ohkuma A, Koya Y, Takahashi H. Hb Fukuoka [beta 2(NA2)His----Tyr]: a new mutation at the 2,3-diphosphoglycerate binding site. Hemoglobin. 1990;14:199-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Farah RA, Buchanan GR, Timmons CF, Phillips L, Fairbanks VF, Snow K, Hoyer JD. Double heterozygosity for Hb G-San Jose [beta7(A4)Glu-->Gly] and Hb Fukuoka [beta2(NA2)His-->Tyr] in a 2 1/2-year-old girl. Hemoglobin. 1999;23:383-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yun YM, Ji M, Ko DH, Chun S, Kwon GC, Lee K, Song SH, Seong MW, Park SS, Song J. Hb variants in Korea: effect on HbA1c using five routine methods. Clin Chem Lab Med. 2017;55:1234-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Xu A, Chen W, Xia Y, Zhou Y, Ji L. Effects of common hemoglobin variants on HbA1c measurements in China: results for α- and β-globin variants measured by six methods. Clin Chem Lab Med. 2018;56:1353-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1583] [Cited by in F6Publishing: 1842] [Article Influence: 460.5] [Reference Citation Analysis (0)] |
| 15. | Ribeiro RT, Macedo MP, Raposo JF. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Curr Diabetes Rev. 2016;12:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |