Published online May 6, 2021. doi: 10.12998/wjcc.v9.i13.3079
Peer-review started: December 22, 2020
First decision: January 10, 2021
Revised: January 15, 2021
Accepted: February 26, 2021
Article in press: February 26, 2021
Published online: May 6, 2021
Hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant genetic disease. Very few patients suffering from HHT present with associated pulmonary arterial hypertension (PAH), which may result in a poor prognosis. Here, we report a case of HHT with PAH. The patient’s clinical manifestations and treatment as well as genetic analysis of family members are reviewed, in order to raise awareness of this multimorbidity.
A 45-year-old Chinese woman was admitted to the hospital to address a complaint of intermittent shortness of breath, which had lasted over the past 2 years. She also had a 30-year history of recurrent epistaxis and 5-year history of anemia. She reported that the shortness of breath had aggravated gradually over the 2 years. Physical examination discovered anemia and detected gallop rhythm in the precordium. Chest computerized tomography and cardiac ultrasound demonstrated PAH and hepatic arteriovenous malformation. The formal clinical diagnosis was HHT combined with PAH. The patient was treated with ambri
We report a novel gene mutation (c. 1232G>A, p. Arg411Gln) in a Chinese HHT patient with PAH.
Core Tip: Hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant genetic disease, which, when associated with pulmonary arterial hypertension (PAH), may result in poor prognosis. As there are many susceptible gene mutations in PAH, there is no clear genetic evidence for HHT with PAH. This is the first report of the activin A receptor-like type 1 c. 1232G>A, p. Arg411Gln mutation in a Chinese HHT patient with PAH. The patient's condition improved obviously after ambrisentan treatment, rather than bosentan. The patient and familial HHT diagnosis was made after the proband’s admission with severe PAH and heart failure. This overdue diagnosis reflects insufficient awareness for HHT diagnosis.
-
Citation: Wu J, Yuan Y, Wang X, Shao DY, Liu LG, He J, Li P. Pulmonary arterial hyper
tension in a patient with hereditary hemorrhagic telangiectasia and family gene analysis: A case report. World J Clin Cases 2021; 9(13): 3079-3089 - URL: https://www.wjgnet.com/2307-8960/full/v9/i13/3079.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i13.3079
Hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant genetic disease, also known as Osler-Weber-Rendu syndrome[1]. The annual incidence rate of this disease is about 1/5000-10000[2,3]. It is divided into type I and type II, according to different underlying pathogenic genes. Type I involves an endoglin (ENG) gene mutation, which leads to abnormal encoding of the ENG glycoprotein. Type II involves an activin A receptor-like type 1 (ACVRL1) gene mutation, which leads to abnormal expression of the encoded activin receptor-like kinase 1 (ALK1). Both mutations disrupt the transforming growth factor (TGF)-β signal transduction pathway and lead to vascular endothelial cell dysgenesis.
Patients with HHT often experience epistaxis, telangiectasia of the skin and mucosa, and arteriovenous malformations (AVMs) of multiple organs. A few patients have presented HHT multimorbidity with pulmonary arterial hypertension (PAH)[4]; these cases have been subcategorized as group 1, based on the comprehensive clinical classification of PAH[5], which seriously affects patient prognosis. The overall etiology remains unknown. The present case report reviews a patient’s clinical manifestations and treatment, as well as the familial genetic analysis, with the aim of raising awareness of the multimorbidity.
A 45-year-old Chinese female patient was admitted to Shengjing Hospital of China Medical University (Shenyang, Liaoning Province, China) with a complaint of intermittent shortness of breath that had begun 2 years prior.
In 2017, the patient had experienced shortness of breath, accompanied by fatigue and palpitations. She described the above symptoms as appearing intermittently and gradually aggravating. In May 2018, she visited an out-patient clinic at the Shengjing Hospital of China Medical University, where a laboratory examination revealed a hemoglobin (Hb) level of 56 g/L (normal range: 130-172 g/L) and serum ferritin level of 4.3 μg/L (normal range: 11.0-336.2 mg/L). She was diagnosed with microcytic hypochromic anemia and was given iron supplement therapy.
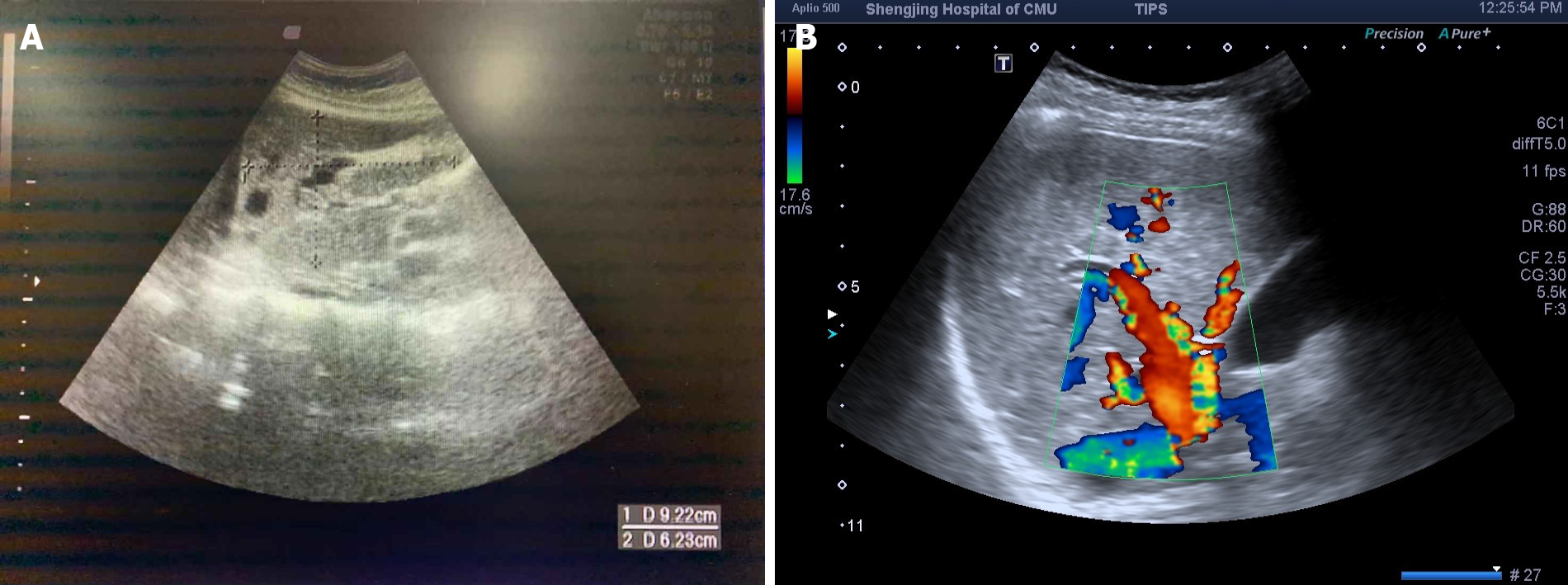
In February 2019, her symptoms of fatigue and shortness of breath became obviously aggravated, with orthopnea and edema affecting both lower limbs. She visited a local hospital, where a laboratory examination revealed an Hb level of 54 g/L, serum iron level of 1.8 mmol/L (normal range: 7-30 mmol/L), serum ferritin level of 4.66 mg/L, and N-terminal pro-B-type natriuretic peptide (referred to as NT-proBNP) level of 918.60 pg/mL (normal range: < 300 pg/mL). Echocardiography showed severe tricuspid regurgitation and PAH, and moderate pericardial effusion (Table 1). Liver ultrasound showed that the hepatic vein and intrahepatic vein branches were widened (Figure 1A).
| February 11, 2019, A | April 16, 2019, B | November 8, 2019, C | |
| ID of aortic root in mm | 30 | 31 | 26 |
| ID of left atrium in mm | 31 | 35 | 36 |
| ID of right ventricle in mm | 43 | 57 | 54 |
| Interventricular septum thickness in mm | 8 | 8 | 8 |
| Left ventricular end diastolic diameter in mm | 49 | 40 | 45 |
| Left ventricular posterior wall thickness in mm | 8 | 8 | 8 |
| ID of pulmonary artery in mm | 33 | 37 | 34 |
| Forward peak velocity in m/s | |||
| Peak E of mitral valve | 1.01 | 0.6 | 0.8 |
| Peak A of mitral valve | 0.8 | 1.2 | |
| Tricuspid valve | 1.3 | 0.8 | |
| Aortic valve | 1.1 | 1.2 | 1.2 |
| Pulmonary valve | 0.96 | 1.0 | 0.8 |
| EDV in mL | 118 | 52 | 127 |
| ESV in mL | 31 | 23 | 58 |
| SV in mL | 87 | 29 | 69 |
| EF, % | 73 | 56 | 55 |
| Peak velocity of tricuspid regurgitation in m/s | 4.1 | 3.9 | 3.6 |
| Pulmonary artery systolic pressure in mmHg | 77 | 75 | 72 |
| Depth of pericardial effusion in mm | 12 | 11-14 | 21 |
| ID of inferior vena cava in mm | 25 | 19 | |
| Variation of inferior vena cava ID with respiration, % | < 20 | < 20 |
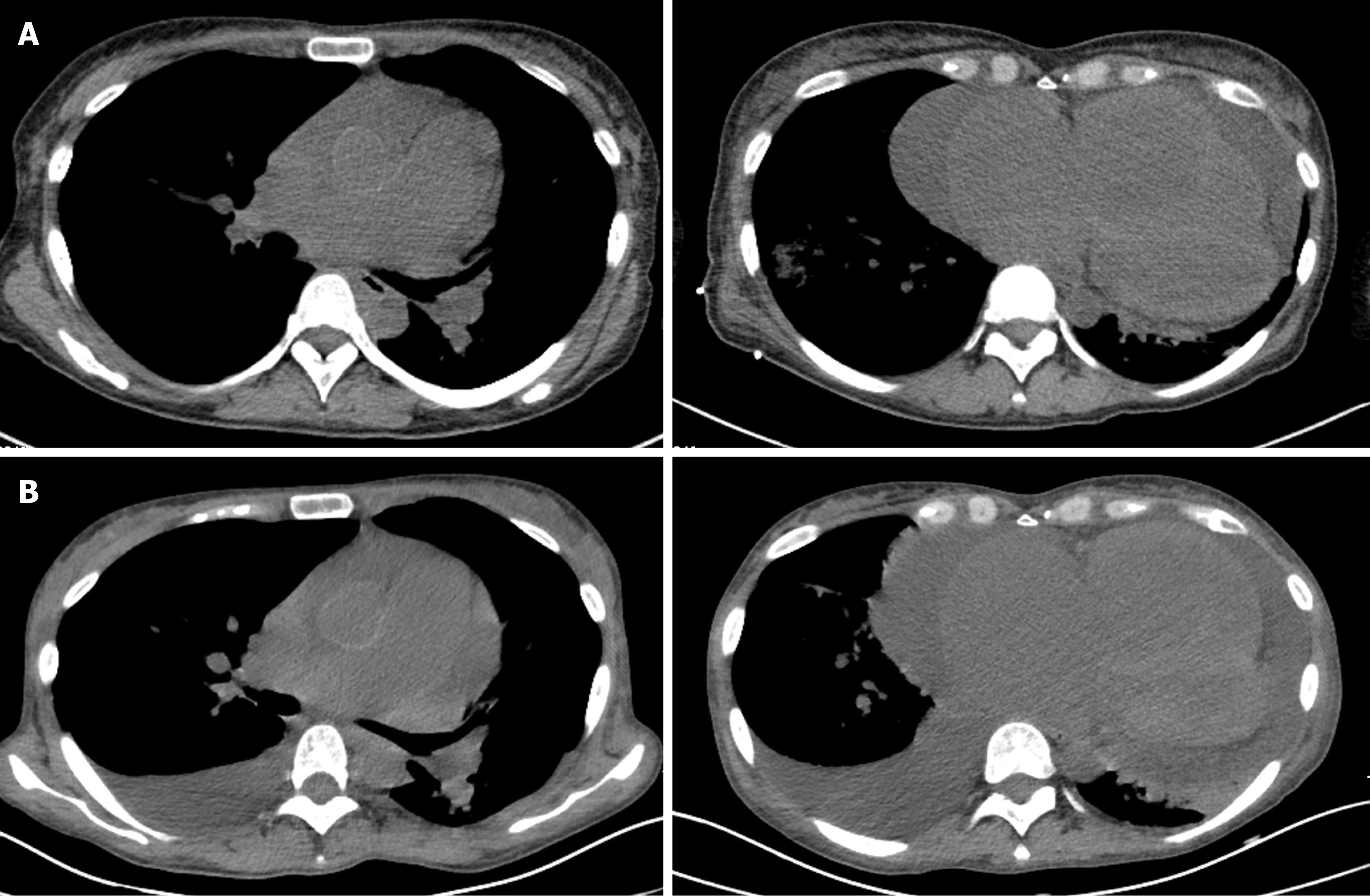
On April 30, 2019, chest computerized tomography (CT) revealed cardiac enlargement, pericardial effusion, and pulmonary artery widening (Figure 2A). Nitrates and diuretics were administered, but the symptoms did not improve significantly. After oral administration of bosentan (125 mg/d for 2 d), the epistaxis symptoms worsened. The patient stopped taking the bosentan and visited our hospital for further treatment. The patient reported experiencing occasional cough with no fever, dizziness, headache, diarrhea, or expectoration. She also reported having poor diet and sleep. Urine volume and body weight were also noted to be decreased.
The patient had a history of recurrent epistaxis for more than 30 years and of anemia for 5 years (having received four blood transfusions). She denied any history of hypertension, diabetes, coronary heart disease, or other chronic diseases.
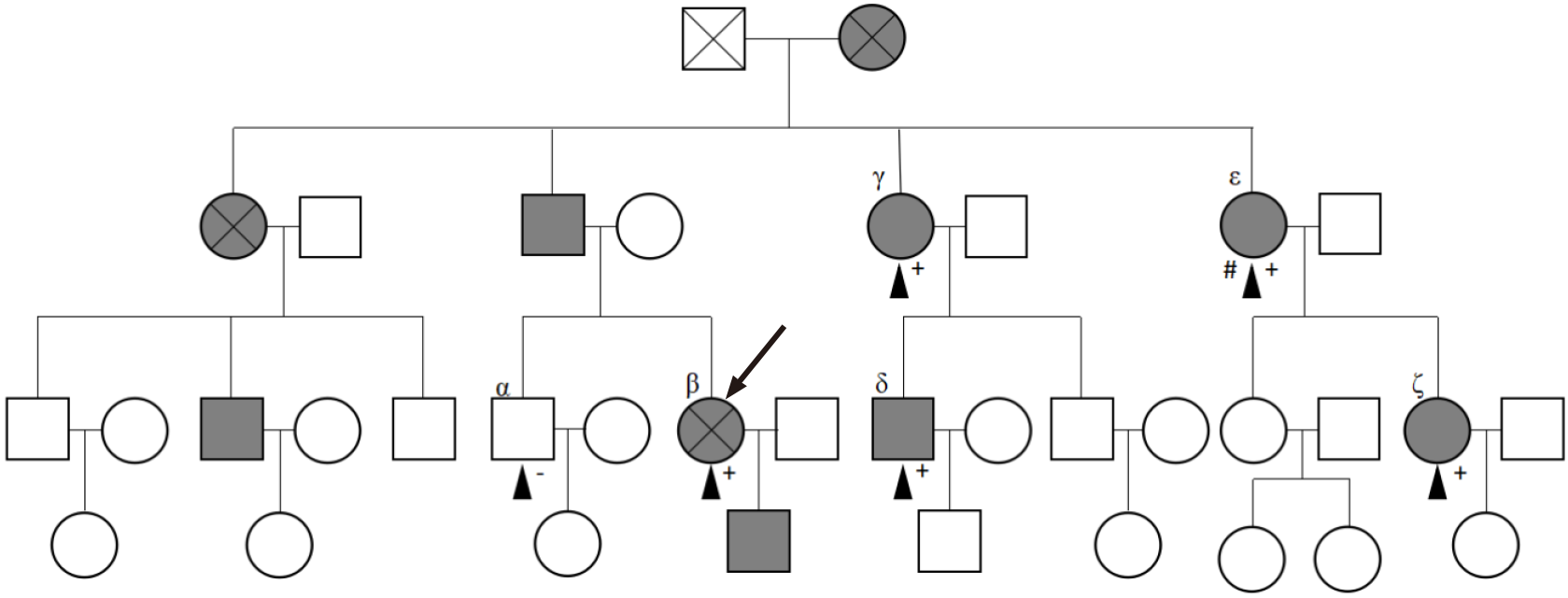
Multiple individuals in the patient’s family had a history of recurrent epistaxis (Figure 3).
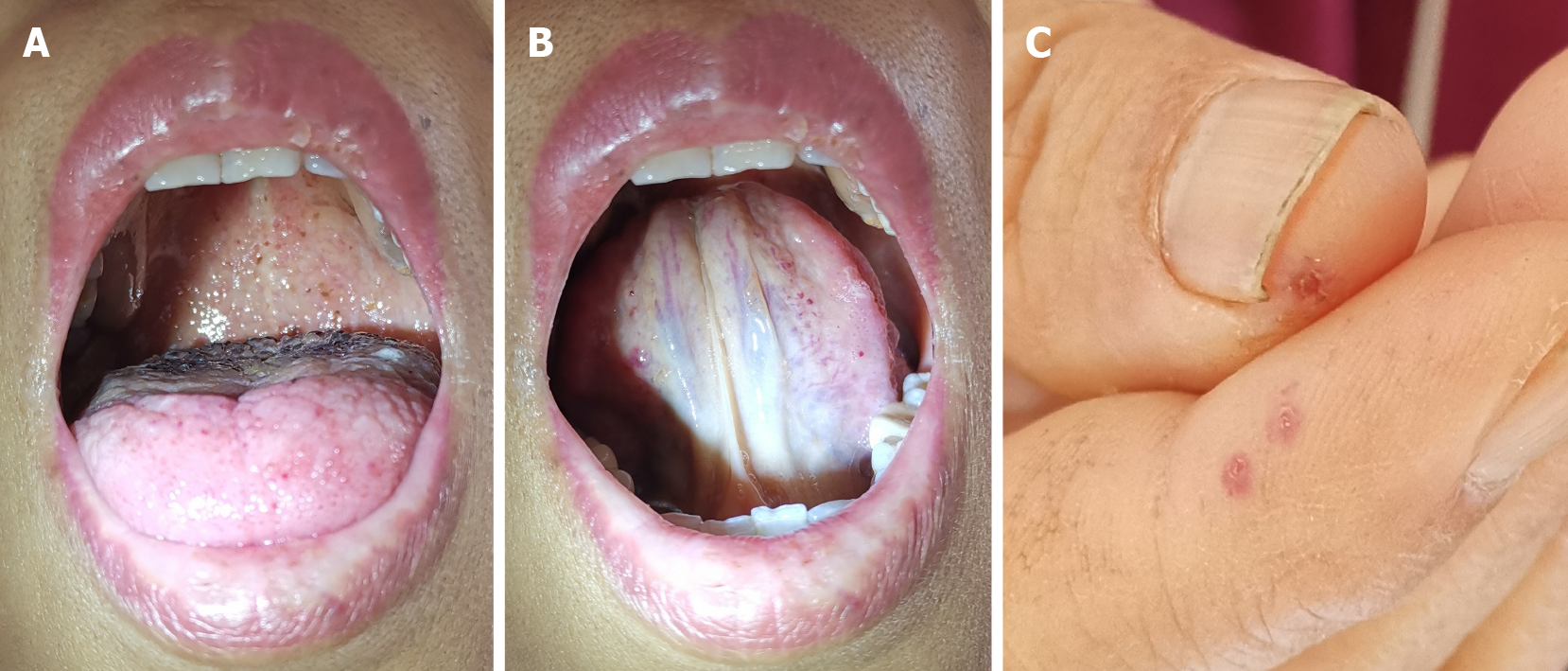
Physical examination upon admission revealed a body temperature of 36.5 °C, blood pressure of 110/61 mmHg, heart rate of 110 beats/min, respiratory rate of 20 breaths/min, oxygen saturation of 93% on air, height of 160 cm, body weight of 60.5 kg, and body surface area of 1.69 m2. The patient had an anemic appearance, with yellow coloration to the skin and scattered capillary dilatation (Figure 4). Her conjunctiva and nail beds were pale, and scattered mucosal capillary dilatation was observed on the tongue (Figure 4). The jugular veins were engorged and pulsating (Video 1). Chest auscultation revealed crude bilateral lung respiratory sounds and no evident dry and moist rales. Cardiovascular examination discovered a gallop rhythm in the precordial region and loud pulmonary valve second heart sound. No marked pathological murmur was detected in the auscultatory valve areas. The abdomen was soft, without tenderness or rebound tenderness. The liver and kidneys were impalpable. Pitting edema was observed in the lower limbs. Pulsation of the dorsalis pedis arteries was decreased.
A routine blood test revealed an Hb level of 81 g/L and hematocrit of 26.95% (normal range: 37%-47%). Blood gas analysis identified a pH value of 7.457 (normal range: 7.35-7.45), partial pressure of oxygen (PaO2) of 66.5 mmHg (normal range: 75-100 mmHg), partial pressure of carbon dioxide (PaCO2) of 25.9 mmHg (normal range: 35-45 mmHg), and oxygen saturation (SaO2) of 91.7% (normal range: 95%-98%). Furthermore, biochemical examination revealed a creatinine level of 77.6 mmol/L, urea nitrogen of 13.06 mmol/L (normal range: 2.5-7.2 mmol/L), D-dimer of 303 mg/L (normal range: 0-252 mg/L), brain natriuretic peptide of 1765.2 pg/mL (normal range: 0-80 pg/mL), albumin of 32.4 g/L (normal range: 35-53 g/L), total bilirubin of 32.7 mmol/L (normal range: 3.4-20.5 mmol/L), creatine kinase (CK) of 1213 U/L (normal range: < 145 U/L), CK-MB of 30 U/L (normal range: < 24 U/L), thyroid stimulating hormone of 11.2123 μIU/mL (normal range: 0.3-4.8 μIU/mL), serum iron of 1.2 mmol/L, and erythropoietin of > 741.00 mIU/mL (normal range: 2.59-18.5 mIU/mL). In addition, troponin was 0.024 mg/L, C-reactive protein was 3.39 mg/L, and NT-proBNP was 2394 pg/mL.
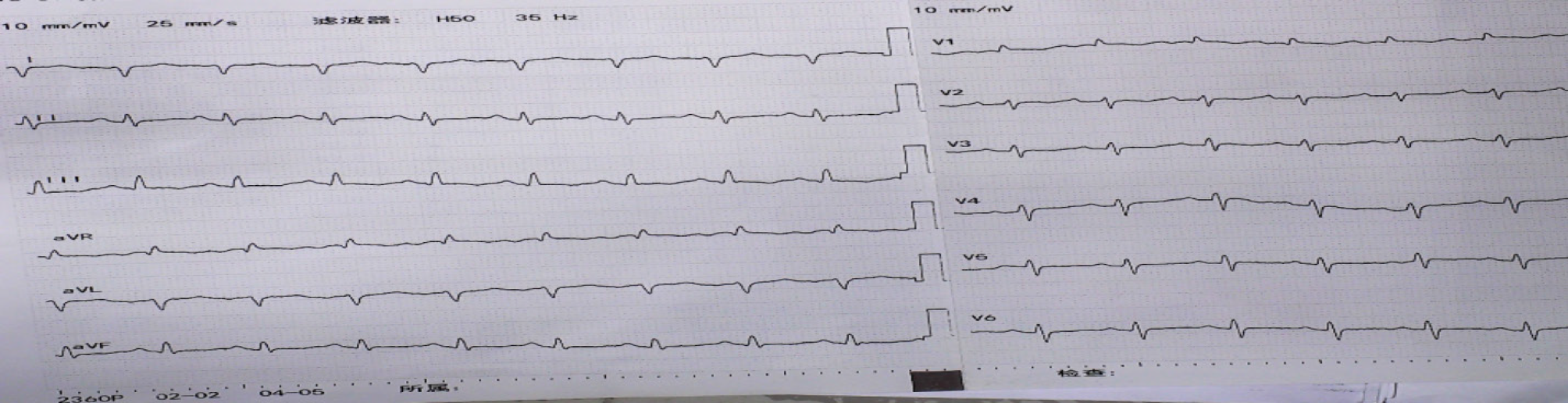
The electrocardiogram (commonly known as ECG) showed the following results: 111 beats/min; V1 R/S > 1; V5 R/S = 1; aVR R/S > 1; sinus tachycardia; possible right ventricular hypertrophy; and lung disease (Figure 5). Cardiac ultrasound (performed on April 16, 2019) revealed right heart enlargement, PAH (moderate to severe), tricuspid regurgitation (moderate to severe), moderate pericardial effusion, widened inferior vena cava with blocked systemic circulation backflow, decreased left ventricular diastolic function, and normal left ventricular systolic function at rest (Table 1). Chest CT (performed on April 30, 2019) showed inflammation in the bilateral lung, with a small amount of left pleural effusion, and enlargement of the heart, with pericardial effusion (Figure 2A).
A final diagnosis of HHT, severe PAH, severe iron deficiency anemia, multiple serous cavity effusion, tricuspid regurgitation (moderate to severe), right heart failure, multiple organ dysfunction syndrome, hypoalbuminemia, and hypoxemia was made. Patient assessment characteristics upon admission were as follows: Grade IV [based on the World Health Organization (WHO) cardiac function grading], Borg dyspnea score of 8, cardiac index of 1.89, high risk (in risk stratification) of adult PAH[6], and APACHE II score of 12.
Treatment administered for the patient’s heart failure included tolvaptan (7.5 mg/d, orally), spironolactone (20 mg/d, orally), digoxin (0.125 mg/d, orally), torasemide (40 mg/d, intravenously), and dobutamine (2 μg/kg/min, continuous intravenous infusion; April 13-23, 2019). But, after 10 d of treatment, the patient’s symptoms were not relieved. In response, ambrisentan was administered orally, at a dose of 10 mg/d. The symptoms of fatigue and shortness of breath improved the day after this treatment was initiated. Intermittent epistaxis was still present during hospitalization, which was relieved by local compression. The bleeding volume and frequency of epistaxis were not significantly different from those before taking ambrisentan.
After 5 d of the ambrisentan administration, a new symptom of cough was noted and the patient began experiencing shortness of breath again. Bedside ultrasound showed right pleural effusion, with a depth of up to 10 cm. Hence, the patient was treated with a 3-d course of right thoracic drainage (April 28 to May 1, 2019); in total, 1305 mL of yellow translucent liquid was drained. The symptoms of cough and shortness of breath were gradually relieved. Chest CT (April 30, 2019) showed a small amount of pleural effusion in the left lung and no effusion shadow in the right (Figure 2A). The drainage tube was removed on May 1, 2019. The patient’s symptoms gradually improved and she was discharged on May 8, 2019, at which point she was able to sleep in a supine position and walk. Her discharge characteristics included the following: WHO cardiac function grade III; Borg dyspnea score of 3; and APACHE II score of 9. Compared to her condition upon admission, the cardiac function grade, Borg dyspnea score, and APACHE II score were improved, although her overall condition remained unsatisfactory. The patient asked to be discharged due to hospitalization expenses.
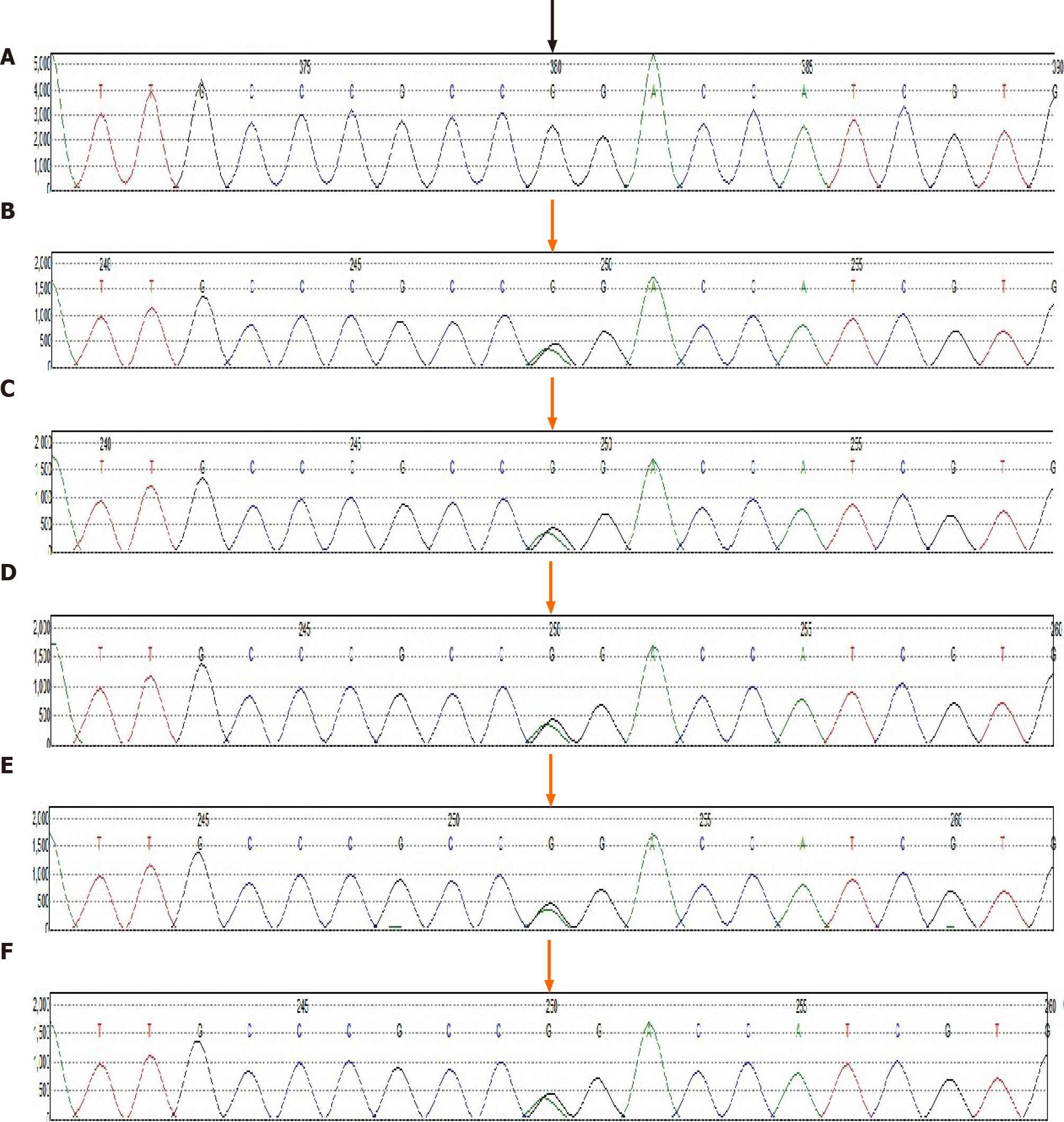
During the hospitalization period, some of the patient’s relatives voluntarily provided their relevant medical history and blood samples for genetic research (Figure 3). Moreover, they agreed to and underwent liver ultrasound, cardiac ultrasound, and ECG examinations. All of the above were approved by the ethics committee of Shengjing Hospital of China Medical University (No. 2019PS510K). Genetic exon mutations (α, β, γ, δ, ε, and ζ) were detected in the family members’ blood samples (Trio family sample test; KingMed Diagnostics, Shenyang, China). An ACVRL1 mutation (12q13.13|NM_000020.2, Exon 8, c. 1232G>A, p. Arg411Gln, heterozygous mutation) was detected in subjects with epistaxis (Figure 6). Liver ultrasound of one family member indicated hepatic AVM (Figure 1B). Except for the proband, there were no obvious abnormalities in cardiac ultrasound and ECG results for the family members.
After discharge, the patient continued to take ambrisentan (10 mg/d) for 6 mo. When the patient developed a fever, shortness of breath, and lower extremity edema, she again presented to our hospital for treatment (November 8, 2019). The patient's vital signs were similar to those upon the last admission for hospitalization. Laboratory examinations revealed an Hb level of 33 g/L, creatinine level of 254.1 μmol/L, and blood potassium level of 6.12 mmol/L (normal range: 3.5-5.5 mmol/L). Cardiac ultrasound showed massive pericardial effusion and blocked systemic circulation backflow (Table 1). Chest CT indicated that the bilateral pleural effusion had increased, especially on the right side (Figure 2B). Abdominal CT showed multiple effusions in the abdomen and pelvis, multiple edematous regions and exudations in the subcutaneous area of the abdominal wall, heterogeneous decrease in the liver parenchyma density, and partial edema in the gastrointestinal tract wall. Dobutamine and furosemide were administered to address the heart failure, and a total of 5 U of red blood cell suspension was infused on an intermittent schedule. However, the patient's condition did not improve and she asked to be discharged from the hospital due to economic reasons. Her family reported (during telephone follow-up after discharge) that the patient had died on November 29, 2019.
The family in this case was definitively diagnosed with type 2 HHT, based on the Curacao standard[7,8]. This diagnosis relied on blood sample analysis of the proband and some relatives, which indicated the presence of an ACVRL1 mutation (c. 1232G>A, p. Arg411Gln) in the family, a history of epistaxis, and presence of capillary dilation of skin and mucosa, and AVM in the liver[9].
No right cardiac catheterization was performed on the proband, due to the seriousness of her condition. However, the patient was diagnosed with severe PAH according to her symptoms (i.e., shortness of breath and edema of lower limbs), physical signs (i.e., P2 hyperfunction and gallop in the precordial area), cardiac ultrasound[10] (showing pulmonary artery diameter > 25 mm, inferior vena cava diameter > 21 mm, tricuspid regurgitation peak velocity > 3.4 m/s, and indirect pulmonary artery systolic pressure > 70 mmHg), and chest CT[11] (showing pulmonary artery diameter of 44 mm and main pulmonary artery diameter/ascending aorta diameter > 1). She was the only patient in this family with HHT and PAH.
To the best of our knowledge, this is the first report of an ACVRL1 mutation (c. 1232G>A, p. Arg411Gln) in a Chinese HHT patient with PAH. Previous Chinese case reports have indicated the same mutation in only one HHT family, in which there was no case complicated with PAH[12]. At present, researchers have found more than 1000 different gene mutations in HHT patients[13], of which 571 are ACVRL1-related (https://arup.utah.edu/database/HHT; last update: January 2018).
Some members of our proband’s family were young, so it was important to accurately predict whether these HHT patients would develop PAH in the future. HHT with PAH is relatively rare, accounting for less than 1% of all HHT cases[4], and there is no clear genetic evidence for this multimorbidity. As there are many susceptible gene mutations in PAH[14], all related mutations were assessed in this family but no specific mutation site was found in the proband (Table 2). Therefore, it was difficult to predict the future occurrence of PAH in HHT-afflicted members of this family[15].
| Pulmonary arterial hypertension-related gene mutation | Mutation site dbSNP | Subjects with the positive gene mutation1 |
| ACVRL1 | rs121909284 | β, γ, δ, ε, ζ |
| rs706815 | α, β, γ, δ, ε, ζ | |
| BMPR2 | rs11390605 | α, β, γ, δ, ε, ζ |
| SMAD4 | rs57847829 | β, γ |
| BMPR1B | rs1365691 | β, δ |
| CAV1 | rs1997623 | α, β, γ, δ, ε, ζ |
| rs2742125 | α, β, γ, δ, ε, ζ | |
| KCNK3 | rs1275920 | α, β, γ, δ, ε, ζ |
| rs1663002 | α, β, γ, δ, ε, ζ | |
| TBX2 | rs35619711 | α, β, γ, δ, ε, ζ |
| rs9891115 | α, β, γ, δ, ε, ζ | |
| rs2240736 | α, β, γ, δ, ε, ζ | |
| rs1057987 | α, β, γ, δ, ε, ζ | |
| TBX4 | rs3744447 | α, β, γ, δ, ε, ζ |
| CBLN2 | rs59751882 | β, ζ |
| rs17853471 | β, ζ | |
| rs7237888 | α, β, γ, δ, ε, ζ | |
| CYP1B1 | rs1056837 | α, β, γ, δ, ε, ζ |
| rs1056836 | α, β, γ, δ, ε, ζ | |
| BAX | rs4645881 | α, β, γ, δ, ε, ζ |
| rs1805419 | α, β, γ, δ, ε, ζ | |
| STAT3 | rs3830585 | α, β, γ, δ |
| rs199748968 | α, β | |
| rs2293152 | α, β, γ, δ, ε, ζ |
Studies have shown that the pathogenic factors for HHT with PAH are directly related to the ACVRL1 and ENG gene mutations (Type I). These two mutations lead to ALK1 and ENG protein abnormalities, which affect the TGF-β signal transduction pathway in vascular endothelial cells[16,17]. This leads to AVMs and pulmonary arteriole occlusion, and reconstruction between dilated arteries and veins[18,19], thereby causing PAH.
HHT combined with PAH belongs to group 1 of the comprehensive clinical classification of PAH[5], which is suitable for the application of endothelin receptor antagonist drugs. In this case, the patient was administered bosentan orally for a short period of time, with withdrawal prompted by the aggravation of epistaxis. After subsequent treatment with ambrisentan, however, there were no aggravating symptoms of epistaxis and the patient’s condition was improved. Compared to bosentan, ambrisentan has a higher frequency of other adverse events, such as nasal congestion[20]; as such, it cannot be determined whether bosentan was the cause of epistaxis aggravation in our patient. Her condition was improved after the ambrisentan treatment, although her pleural effusion was increased. At present, there is no evidence for the increase of pleural effusion caused by ambrisentan. Therefore, the increase in pleural effusion in this case could be related to PAH and heart failure, rather than the treatment.
Multiple generations of the patient’s many family members have suffered from recurrent epistaxis for decades. The HHT diagnosis was made for this family after the proband’s admission to our hospital, as she was suffering from severe PAH and heart failure. This overdue diagnosis reflects the clinicians’ inability to properly diagnose HHT. Thus, it is necessary to cultivate the knowledge of this disease, with the aim of avoiding misdiagnosis and improving HHT patient prognosis[21]. The prognosis itself will be improved if the proband can be diagnosed with HHT with PAH, and treated with an endothelin receptor antagonist in the early stages of the disease.
HHT with PAH is a rare multimorbidity. The ACVRL1 mutation (c. 1232G>A, p. Arg411Gln) was the cause of such for the case presented herein, representing the first report of a Chinese HHT patient with PAH. HHT diagnosis should be considered for patients with PAH if epistaxis, telangiectasia of the skin and mucous membranes, or visceral AVMs are present. HHT with PAH can be treated with endothelin receptor antagonists and genetic tests should be carried out in the patient’s family. More studies of these rare cases are needed for clinicians to further understand this multimorbidity.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krishnan A S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Blanchet AS, Cottin V, Cordier JF. [Pulmonary vascular manifestations in hereditary hemorrhagic telangiectasia]. Presse Med. 2005;34:1487-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, Gossage JR, Guttmacher AE, Hyland RH, Kennedy SJ, Korzenik J, Mager JJ, Ozanne AP, Piccirillo JF, Picus D, Plauchu H, Porteous ME, Pyeritz RE, Ross DA, Sabba C, Swanson K, Terry P, Wallace MC, Westermann CJ, White RI, Young LH, Zarrabeitia R; HHT Foundation International - Guidelines Working Group. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 652] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 3. | Lacombe P, Lacout A, Marcy PY, Binsse S, Sellier J, Bensalah M, Chinet T, Bourgault-Villada I, Blivet S, Roume J, Lesur G, Blondel JH, Fagnou C, Ozanne A, Chagnon S, El Hajjam M. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: An overview. Diagn Interv Imaging. 2013;94:835-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Cottin V, Plauchu H, Dupuis-Girod S, Cordier JF. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia: follow-up and pathophysiologic considerations. J Vasc Interv Radiol. 2007;18:938-9; author reply 939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3636] [Cited by in F6Publishing: 3916] [Article Influence: 435.1] [Reference Citation Analysis (0)] |
| 6. | Hoeper MM, Pittrow D, Opitz C, Gibbs JSR, Rosenkranz S, Grünig E, Olsson KM, Huscher D. Risk assessment in pulmonary arterial hypertension. Eur Respir J. 2018;51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 8. | Sharathkumar AA, Shapiro A. Hereditary haemorrhagic telangiectasia. Haemophilia. 2008;14:1269-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Cazacu IM, Singh BS, Saftoiu A, Bhutani MS. Recent developments in hepatopancreatobiliary EUS. Endosc Ultrasound. 2019;8:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Bossone E, D'Andrea A, D'Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 11. | Freed BH, Collins JD, François CJ, Barker AJ, Cuttica MJ, Chesler NC, Markl M, Shah SJ. MR and CT Imaging for the Evaluation of Pulmonary Hypertension. JACC Cardiovasc Imaging. 2016;9:715-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Lin WD, Wu JY, Hsu HB, Tsai FJ, Lee CC, Tsai CH. Mutation analysis of a family with hereditary hemorrhagic telangiectasia associated with hepatic arteriovenous malformation. J Formos Med Assoc. 2001;100:817-819. [PubMed] [Cited in This Article: ] |
| 13. | HHT Mutation Database-University of Utah. Hereditary Hemorrhagic Telangiectasia. [cited 12 January 2020]. Available from: http://arup.utah.edu/database/HHT/. [Cited in This Article: ] |
| 14. | Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, Humbert M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D13-D21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 15. | Girerd B, Montani D, Coulet F, Sztrymf B, Yaici A, Jaïs X, Tregouet D, Reis A, Drouin-Garraud V, Fraisse A, Sitbon O, O'Callaghan DS, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med. 2010;181:851-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Alkhormi AM, Memon MY, Alqarawi A. Gastric Antral Vascular Ectasia: A Case Report and Literature Review. J Transl Int Med. 2018;6:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Pousada G, Baloira A, Valverde D. Pulmonary arterial hypertension and portal hypertension in a patient with hereditary hemorrhagic telangiectasia. Med Clin (Barc). 2015;144:261-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Peng X, Wei C, Li HZ, Li HX, Bai SZ, Wang LN, Xi YH, Yan J, Xu CQ. NPS2390, a Selective Calcium-sensing Receptor Antagonist Controls the Phenotypic Modulation of Hypoxic Human Pulmonary Arterial Smooth Muscle Cells by Regulating Autophagy. J Transl Int Med. 2019;7:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Sawant KV, Xu R, Cox R, Hawkins H, Sbrana E, Kolli D, Garofalo RP, Rajarathnam K. Chemokine CXCL1-Mediated Neutrophil Trafficking in the Lung: Role of CXCR2 Activation. J Innate Immun. 2015;7:647-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Men W, Xiao H, Yang Z, Fan D. Agglomeration Effect of Medical Education: Based on the Web of Science Database. J Transl Int Med. 2018;6:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |