Published online Aug 26, 2020. doi: 10.12998/wjcc.v8.i16.3534
Peer-review started: April 24, 2020
First decision: May 15, 2020
Revised: June 8, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: August 26, 2020
Processing time: 123 Days and 3.4 Hours
Extrahepatic metastasis (EHM) from hepatocellular carcinoma (HCC) occurs in 10%–15% of cases following initial treatment. The most frequent sites of EHM are the lung, lymph nodes, and bone. Gastrointestinal or brain metastasis from HCC is rarely reported. Here, we report a rare case of concurrent HCC metastases to the stomach, colon, and brain.
A 72-year-old male with a history of alcohol induced HCC presented with multiple intrahepatic recurrences and tumorous lesions in the stomach and ascending colon. Three years earlier, he underwent right hemihepatectomy, and 1 year ago, he had a video-assisted thoracoscopic wedge resection for pulmonary metastasis of HCC. We decided on surgical resection of the new metastases because of massive gastric bleeding and concern for possible colonic obstruction. The patient underwent gastric wedge resection and right hemicolectomy. Two weeks later, the patient developed dysarthria and mild cognitive disorder. Magnetic resonance imaging of the brain revealed a left frontal lobe lesion, and he underwent resection of a metastatic brain tumor. Unfortunately, he died 6 weeks after the last surgery due to hepatorenal syndrome.
Decision of surgery was carefully recommended in this case and may extend survival in other metastatic HCC patients with well-preserved hepatic function.
Core tip: This was a very unusual case of concurrent stomach, colon, and brain metastasis of hepatocellular carcinoma. Extrahepatic metastasis of hepatocellular carcinoma has a poor prognosis. However, deteriorated hepatic function due to intrahepatic metastases is more often the cause of death than is extrahepatic metastasis. Treatment of extrahepatic metastases is still suggested to control tumor progression. Surgical resection of these metastases may extend survival and quality of life and should be considered in patients with well-preserved hepatic function.
- Citation: Kim R, Song J, Kim SB. Concurrent hepatocellular carcinoma metastasis to stomach, colon, and brain: A case report. World J Clin Cases 2020; 8(16): 3534-3541
- URL: https://www.wjgnet.com/2307-8960/full/v8/i16/3534.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i16.3534
Extrahepatic metastasis (EHM) from hepatocellular carcinoma (HCC) occurs in 10%–15% of cases of recurrence following initial treatment[1-6]. The most frequent sites of EHM are the lung, lymph nodes, and bone[1-7]. Hematogenous and lymphatic spreading patterns seem to be the most important mechanisms of EHM[5].
Gastrointestinal metastasis is rarely reported for HCC[6-9]. In one autopsy study, there was just one case each of gastric or colonic metastasis from HCC[6]. Another recent case reported HCC metastasis to the ascending colon and stomach[8].
The incidence of brain metastases from HCC is also extremely rare, with a reported incidence of 0.2%-2.2%[10,11]. Because of this rarity, the prognosis and treatment strategies for patients with HCC brain metastases are still under investigation[10]. Here, we report the first known case of synchronous HCC metastases to the stomach, colon, and brain.
A 75-year-old male with a history of alcoholic liver cirrhosis and HCC presented to our hospital for transarterial chemoembolization (TACE). He complained of melena and mild dyspnea.
Patients reported intermittent melena episodes 1 wk ago and worsened over two to 3 d.
The patient underwent right hemihepatectomy for HCC 3 years ago. One year ago, he had a left lower lung wedge resection for pulmonary metastasis of HCC. 5 mo ago, he had multiple intrahepatic HCC recurrence and underwent transarterial chemoembolization (TACE). The gastric and colonic metastases were not prominent radiologic findings at the time. 4 mo ago, other intrahepatic recurrences developed and intestinal lesions were prominent.
The patient had diabetes and hypertension. There was no family history of cancer.
Hypotension and tachycardia were present at admission, and the patient recovered after hydration and transfusion. The abdominal was soft and tenderness or rebound tenderness was not observed. The rectal examination showed melena, but no hematochezia. 2 wk after gastrectomy and colectomy, he complained of dysarthria and mild cognitive disorder.
The hemoglobin level was 7.1 g/dL and recovered to 9.4 g/dL after two RBC transfusions. The prothrombin time (international normalized ratio, INR) was 1.04 (normal range 0.86–1.14). The total bilirubin was 0.3 mg/dL (normal range 0.3 –1.2), and the albumin was 2.8 g/dL (normal range 3.5–5.2). The aspartate aminotransferase was 27 U/L (normal range < 35), and the alanine aminotransferase was 26 U/L (normal range < 45). The serum levels of protein induced by vitamin K absence or antagonist II (PIVKA-II) and α-fetoprotein were not elevated, at 15 mAU/mL (normal range < 40 mAU/mL) and 2.3 ng/mL (normal range < 7.0 ng/mL), respectively.
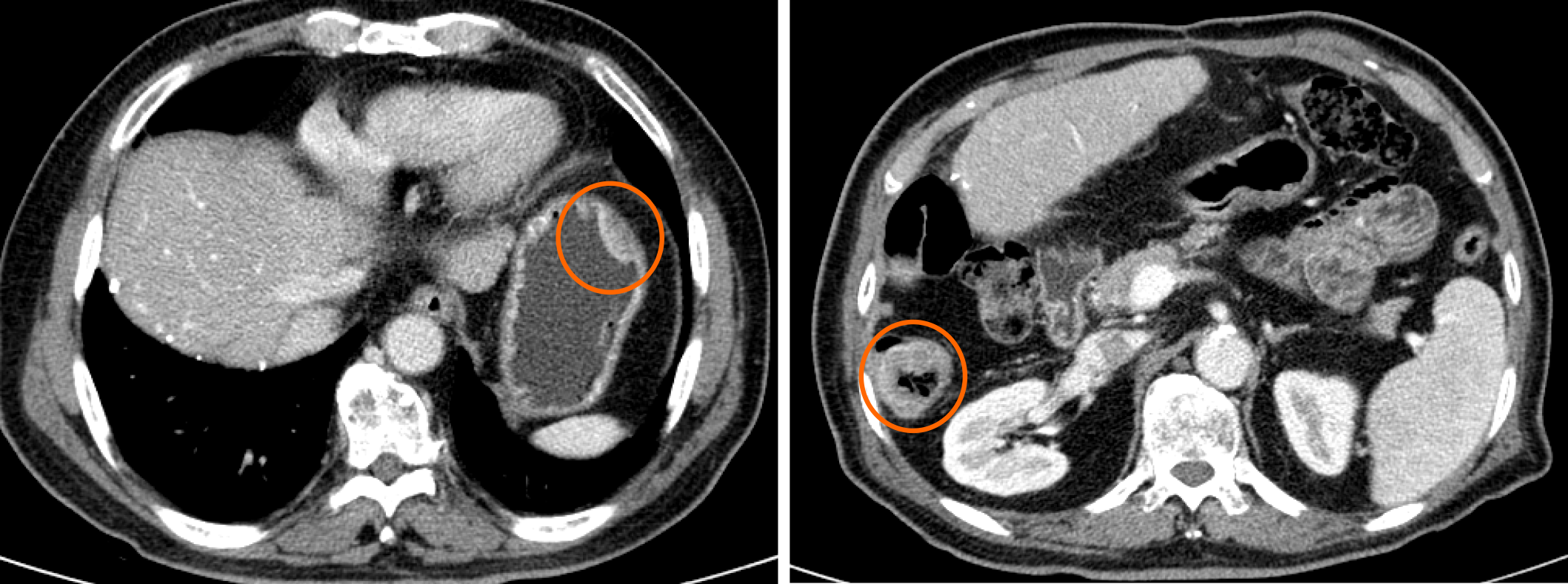
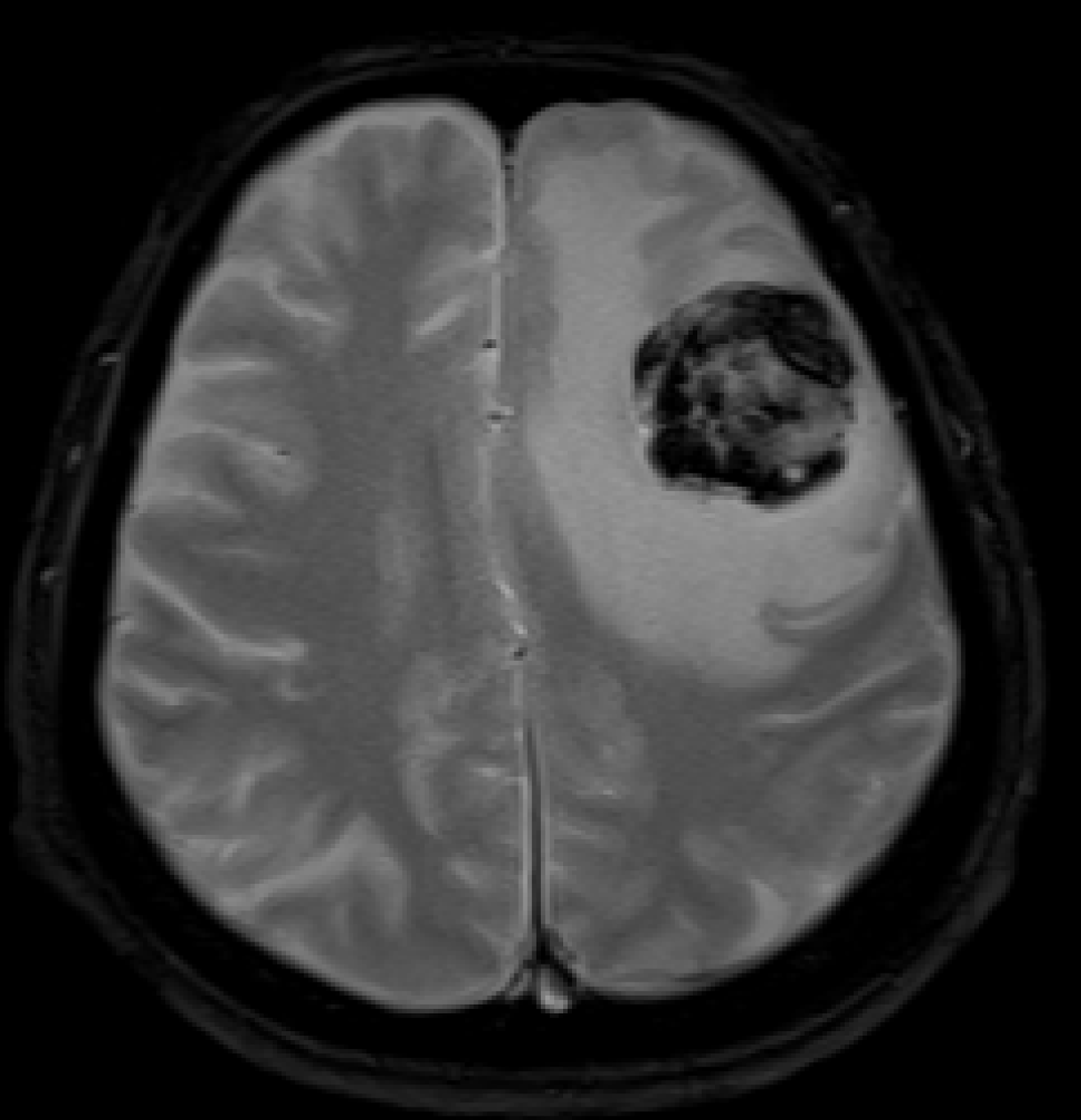
A computed tomography (CT) scan showed intramural wall thickening of the stomach body and luminal narrowing of the ascending colon (Figure 1). Magnetic resonance imaging (MRI) of the brain 2 wk after abdominal surgery showed a 38 mm lesion in the left frontal lobe with hemorrhagic features (Figure 2).
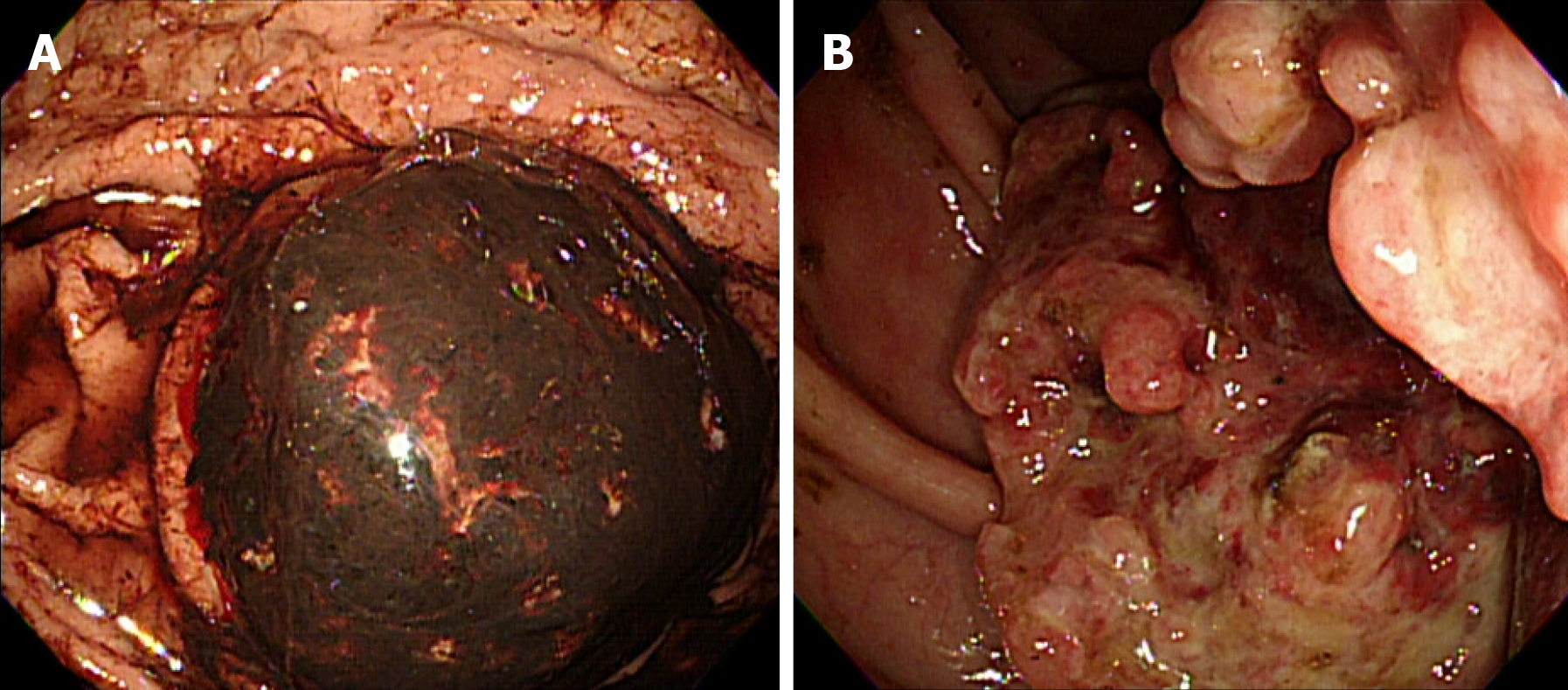
Endoscopy and colonoscopy revealed a bleeding, fungating mass in the lesser curvature of the stomach body, and an ulcerofungating tumor of the ascending colon that was suspicious for partial obstruction. Biopsies of both lesions confirmed metastatic HCC (Figure 3).
The patient was diagnosed with concurrent metastatic HCC of the stomach, colon, and brain.
Although the optimal treatment option was sorafenib, we decided to perform surgical resection because of massive gastric bleeding and concern for possible colonic obstruction. The patient underwent gastric wedge resection and right hemicolectomy. 2 wk later, he underwent brain tumorectomy.
The postoperative course after abdominal surgery was uneventful. After the brain operation, his cognitive impairment and dysarthria recovered. Ten days after the brain surgery, his urinary output was decreased and weight gain had developed. The size and number of intrahepatic recurrent tumors were slightly increased, and a large number of ascites were detected by CT. Despite the use of diuretics, the control of ascites became difficult and the azotemia aggravated. We recommended dialysis to the patient, but the patient refused further treatment. Unfortunately, the patient suffered hepatorenal syndrome after brain surgery and died 6 wk after the brain operation.
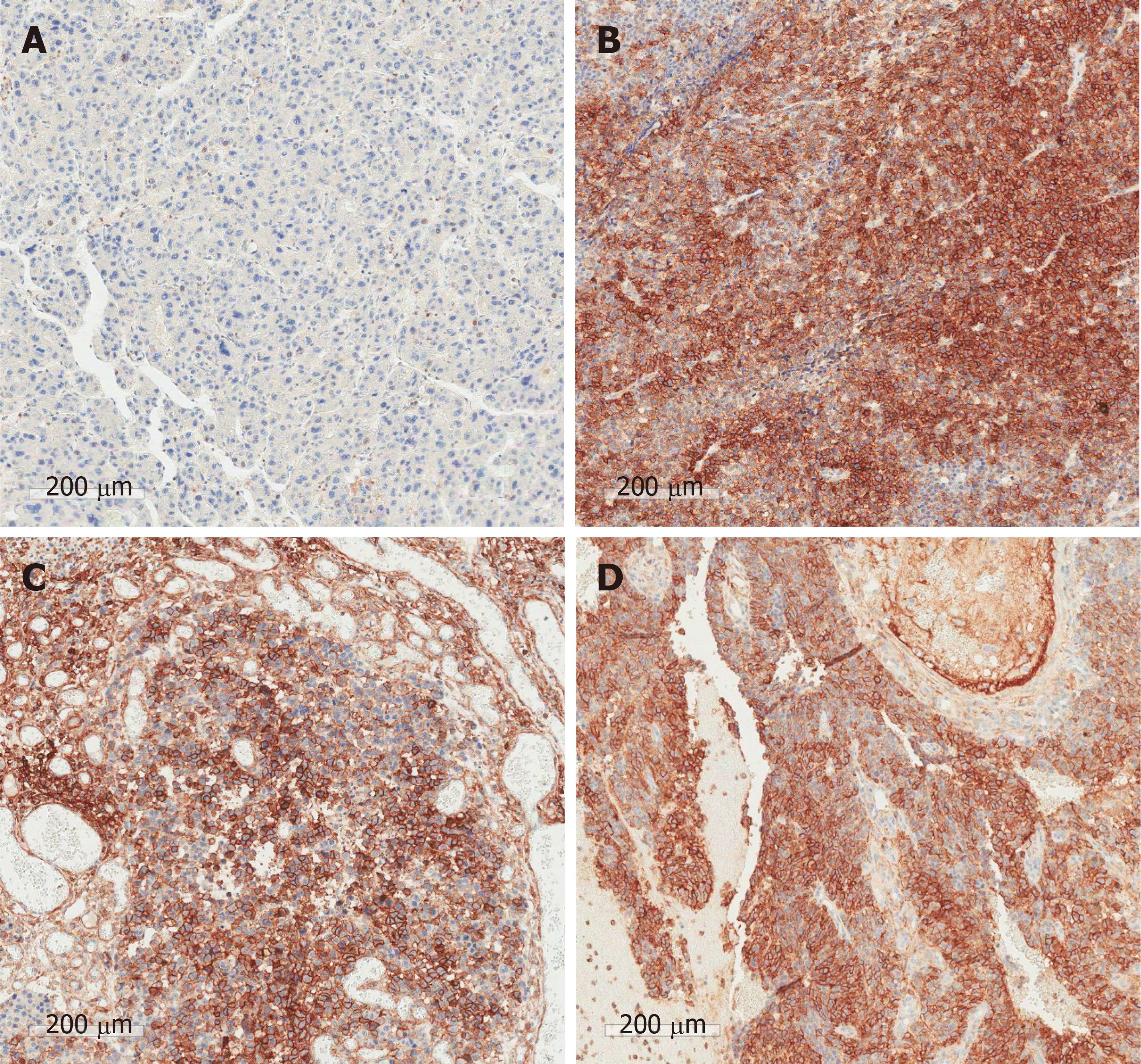
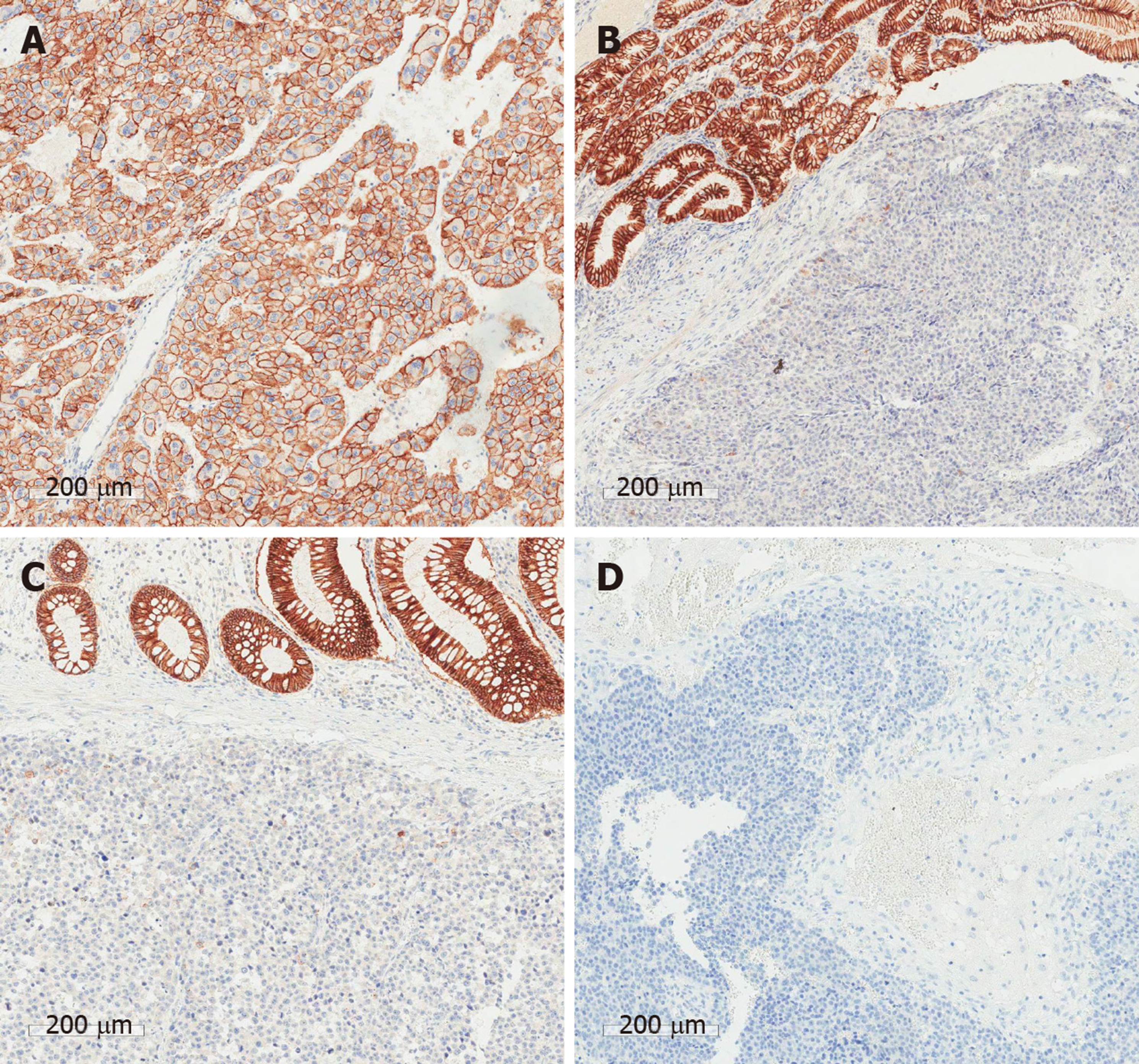
The pathologic findings of the gastric and colonic specimens showed metastatic HCC with mucosal to serosal invasion, without involvement of adjacent lymph nodes. Analysis of the brain specimen showed metastatic HCC with hemorrhage invading the normal brain issue. In the immunohistochemical analyses between the primary liver tumor and all metastatic lesions, CD44, p53, and Ki-67 were strongly positive in the metastatic tissues. E-cadherin, glypican-3 (GPC-3), and hepatocyte paraffin 1 (HepPar1) were positive in the primary tumor tissue. Vimentin was negative in both the primary tumor and metastatic lesions. (Figure 4 and Figure 5 and Table 1).
| Markers | Primary liver | Stomach | Colon | Brain |
| HepPar1 | P | N | N | N |
| p53 | N | P | P | P |
| Ki-67 | 5% | 60% | 60% | 60% |
| E-cadherin | P | N | N | N |
| Vimentin | N | N | N | N |
| GPC-3 | P | N | WP | N |
| CD44 | N | P | P | P |
Extrahepatic metastasis from HCC has extremely poor prognosis[1-5,12]. However, extrahepatic progression is reported as the cause of death in less than 10% of the patients with EHM from HCC[1-6]. Rather, the control of intrahepatic tumor lesions is the prognostic factor in these cases[1-3,12]. In fact, deteriorated hepatic function was strongly considered as a cause of death in the present case. Although tumor factors at the time of hepatectomy strongly influence prognosis, treatment of EHM is important to delay extrahepatic tumor progression[2]. A surgical approach should be considered for patients with resectable extrahepatic metastasis from HCC.
Cheng et al[7] reviewed 11 cases of gastrointestinal metastasis from HCC. In their study, the initial presentations were massive bleeding from the GI tract and epigastric discomfort. Most patients presented with large tumor size and high tumor marker levels. The metastatic pattern of most cases was direct invasion from the subserosal to mucosal layer. In contrast, recent GI metastasis cases were described with the hematogenous spread pattern rather than direct invasion[8,9]. In our patient, the metastatic pattern was hematogenous spread without surrounding lymph node involvement. Most reports[7-9] indicate that patient’s symptoms, radiologic findings, and elevated tumor markers were important for the detection of GI metastasis. However, tumor markers in our case were not elevated at the time the GI metastases were detected. Therefore, detailed radiologic interpretation and annual endoscopic evaluation are advisable for HCC patient follow up.
Almost all patients with brain metastases present with neurologic symptoms such as headache, motor weakness, aphagia, ataxia, blurred vision or hemiparesis/ hemiplegia[10,11]. Our patient complained of cognitive disorder and dysarthria, without headache or motor weakness. Patients with EHM from HCC who show any neurologic disorder should have radiologic evaluation with a neurologic specialist. In addition, most patients with HCC brain metastases also present with simultaneous pulmonary metastases. In fact, pulmonary metastasis may be a key sign of brain metastases and should be an indication for brain CT[10-12]. After assessment for brain metastasis, further evaluation for other extrahepatic metastases also should be considered.
The tumor biology between the primary HCC and metastatic lesions showed different characteristics. It showed the possibility of an HCC primary tumor acquiring a new metastatic mechanism due to stimulation by the tumor microenvironment. E-cadherin repression is essential for disassembling intercellular junctions, which is known to facilitate hematogenous metastasis[16]. CD44, as a cancer stem cell marker, is stimulated by the mesenchymal markers[13,14]. Epithelial-mesenchymal transition is also a key mechanism of hematogenous metastasis. The variation of numerous proteins in the metastasis of HCC is one reason why therapy targeted to specific proteins is not effective. It might be the reason that multi-kinase inhibitors, such as sorafenib and lenvatinib, had efficacious results for the patient with HCC metastasis. Therefore, it is important to study the characteristics of tumors and plan the treatment direction by considering differences between metastatic lesions and the primary tumor.
In brain metastases of various tumors, angiogenesis was identified as an important mechanism, demonstrating the possibility of neo-angiogenetic therapy[17]. There are two case reports using sorafenib in brain metastasis[18,19]. Sorafenib treatment for patients with brain metastases from thyroid cancer or renal cell carcinoma increased survival by more than one year. However, these cases were not hepatic metastases and liver function was normal. In addition, the improvement of symptoms after treatment took more than 1 mo. There is still a lack of randomized controlled trial studies for neo-angiogenetic therapy for brain metastasis. Therefore, comparing the therapeutic effects of surgical treatment with chemotherapy in brain metastases still requires further study.
The most common cause of death in EHM from HCC is not progression of EHM, but hepatic functional deterioration by recurrent intrahepatic HCC. Although our patient died due to hepatorenal syndrome 6 wk post-surgery, we cautiously suggest that a surgical approach for EHM could result in longer survival and improved quality of life in selected patients with well-maintained hepatic function.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe H S-Editor: Wang DM L-Editor: A P-Editor: Wang LL
| 1. | Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475-4483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 3. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 4. | Kanda M, Tateishi R, Yoshida H, Sato T, Masuzaki R, Ohki T, Imamura J, Goto T, Yoshida H, Hamamura K, Obi S, Kanai F, Shiina S, Omata M. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Chua TC, Morris DL. Exploring the role of resection of extrahepatic metastases from hepatocellular carcinoma. Surg Oncol. 2012;21:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Terada T, Maruo H. Unusual extrahepatic metastatic sites from hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6:816-820. [PubMed] |
| 7. | Sano T, Izuishi K, Takebayashi R, Akamoto S, Kakinoki K, Okano K, Masaki T, Suzuki Y. Surgical approach for extrahepatic metastasis of HCC in the abdominal cavity. Hepatogastroenterology. 2011;58:2067-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Wu D, Wei S, Liu B, Wu X, Feng Y, Luo C, Ju Y, Liang J. Effect of immune suppression on metastasis in a patient with hepatocellular carcinoma metastasized to the colon and stomach: A case report. Exp Ther Med. 2016;11:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Mitsialis V, Lee LS. Metastasis of Hepatocellular Carcinoma to Distal Colon Associated With Inferior Mesenteric Arteriovenous Fistula and Tumor Thrombus: a Case Report. Am J Gastroenterol. 2018;113:916-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Jiang XB, Ke C, Zhang GH, Zhang XH, Sai K, Chen ZP, Mou YG. Brain metastases from hepatocellular carcinoma: clinical features and prognostic factors. BMC Cancer. 2012;12:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Han JH, Kim DG, Park JC, Chung HT, Paek SH, Chung, YS. Little response of cerebral metastasis from hepatocellular carcinoma to any treatments. J Korean Neurosurg Soc. 2010;325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, Choi JY, Yoon SK, Chung KW, Kay CS, Jung HS. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012;27:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Zhong J, Chen Y, Wang LJ. Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol. 2016;22:2434-2440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Yan Y, Zuo X, Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med. 2015;4:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 473] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 15. | Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu X, Sun J, Yang S, Poon RT, Fan ST. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int J Cancer. 2012;131:E163-E172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Chen J, Zhao J, Ma R, Lin H, Liang X, Cai X. Prognostic significance of E-cadherin expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e103952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Berghoff AS, Preusser M. Anti-angiogenic therapies in brain metastases. Memo. 2018;11:14-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Shen Y, Ruan M, Luo Q, Yu Y, Lu H, Zhu R, Chen L. Brain metastasis from follicular thyroid carcinoma: treatment with sorafenib. Thyroid. 2012;22:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Hu D, Hu Y, Li J, Wang X. Symptomatic treatment of brain metastases in renal cell carcinoma with sorafenib. J Cancer Res Ther. 2018;14:S1223-S1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |