Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2876
Peer-review started: January 26, 2020
First decision: February 26, 2020
Revised: March 26, 2020
Accepted: June 7, 2020
Article in press: June 7, 2020
Published online: July 6, 2020
Pulmonary sarcomatoid carcinoma (PSC), a rare subtype of non-small cell lung cancer (NSCLC), is poorly differentiated and highly aggressive. Treatment is limited, and the prognosis is poor. Pembrolizumab is an anti-programmed death (PD)-1 antibody with good efficacy in NSCLC. Recent studies have demonstrated that PD-ligand 1 (PD-L1) overexpression is common in PSCs, which suggests that anti-PD-L1 treatment is an ideal option. However, the response to pembrolizumab in PSC has not been studied.
We present a PSC case with PD-L1 overexpression that significantly benefited from pembrolizumab. A 73-year-old Chinese male was detected with a right lung lesion. Pathological analysis of the right upper lobectomy confirmed PSC. The PD-L1 test revealed overexpression (TPS: 90%). Multiple metastases occurred 1 mo after surgery, representing stage IV PSC. Neither first-line chemotherapy nor second-line antiangiogenic agents showed any benefit. Radiotherapy (1200 cGy) was administered to relieve chest wall pain. The patient received the PD-1 inhibitor pembrolizumab (100 mg) as third-line therapy; however, because of fever and severe infection, he refused to receive immunotherapy any longer. Thus, only one dose of pembrolizumab was administered. Deep sustained remission of most of the metastases was achieved except for lesions in the right adrenal gland, which first shrank and then progressed. The patient died because of disease progression in the right adrenal gland. He achieved a progression-free survival time of 8 mo and an overall survival time of 9 mo with third-line pembrolizumab.
Our findings highlight and offer direct evidence of the efficacy of pembrolizumab in PD-L1-overexpressing PSCs. Combined radiotherapy and immunotherapy may enhance treatment efficacy.
Core tip: This is a report of a patient with programmed death-ligand 1 (known as PD-L1)-overexpressing pulmonary sarcomatoid carcinoma with a good response to pembrolizumab, indicating that pembrolizumab is an important treatment for pulmonary sarcomatoid carcinoma patients with PD-L1 overexpression. In this case, the patient received low-dose radiotherapy before pembrolizumab, which suggests that the combination of radiotherapy and immunotherapy may elevate treatment efficacy.
- Citation: Chen P, Yu M, Zhang JL, Chen WY, Zhu L, Song Y, Jiang CY, Zhang S. Significant benefits of pembrolizumab in treating refractory advanced pulmonary sarcomatoid carcinoma: A case report. World J Clin Cases 2020; 8(13): 2876-2884
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2876
Pulmonary sarcomatoid carcinoma (PSC) comprises a rare group of non-small cell lung cancer (NSCLC). According to the Surveillance, Epidemiology, and End Results database, PSC accounts for 0.52% of all NSCLC cases[1]. PSC is characterized by poorly differentiated, highly aggressive, and highly metastatic properties, and its prognosis is much poorer than that of other NSCLC subtypes[2,3]. Moreover, PSCs are not sensitive to conventional chemotherapy[4,5]; thus, developing novel therapeutic strategies is essential. Programmed death 1 (PD-1) and PD-ligand 1 (PD-L1) inhibitors have clinical efficacy in NSCLC[6-8]. In general, the efficacy of immunotherapy parallels the level of PD-L1 expression. Recently, studies have demonstrated that PD-L1 overexpression is common in PSCs, ranging from 53% to 69.2%[9-12], which makes immunotherapy a promising treatment option for PSCs. However, studies of immunotherapy in PSC are very limited to date, and only a few reports can be found[13-15]. Here, we present one patient with PSC, who developed deep sustained remission for most metastatic lesions except for right adrenal lesions, after treatment with one dose of the PD-1 inhibitor pembrolizumab.
A 73-year-old Chinese male patient was initially admitted to the hospital due to a space-occupying lesion in the right lung found during a routine health examination.
The patient did not experience any symptoms or discomfort before this examination.
The patient had a clear medical history.
The patient had a long-term smoking history for approximately 45 years (20 cigarettes per day) without quitting until the disease was detected. He had no personal or family history of other diseases.
At admission, the patient was conscious, body temperature was 36.3 °C, with a regular heart rate of 68 bpm, respiratory rate of 16 breaths per minute, and blood pressure of 120/70 mmHg. He reported no history of weight loss during recent months. The patient’s Eastern Cooperative Oncology Group (referred to as ECOG) Performance Status (PS) score was 0. His right upper lung breathing sounds were weak. The other physical examinations were normal.
The results of routine laboratory tests including routine blood examination, blood biochemistry, routine urine examination, fecal occult blood, and tumor markers were all within normal limits.
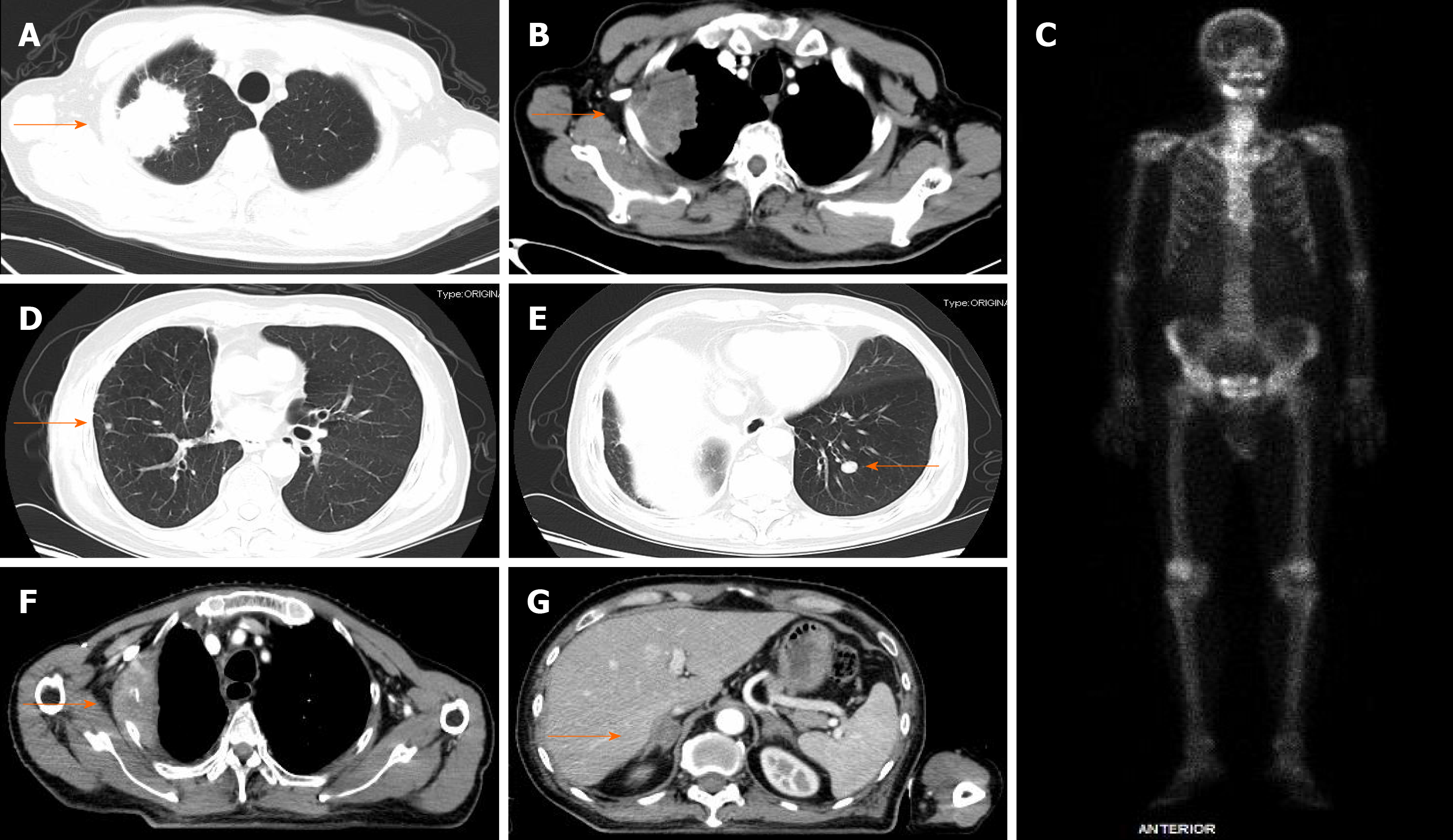
Computed tomography (CT) of the chest revealed a space-occupying lesion in the apical segment of the upper lobe of the right lung (Figure 1).
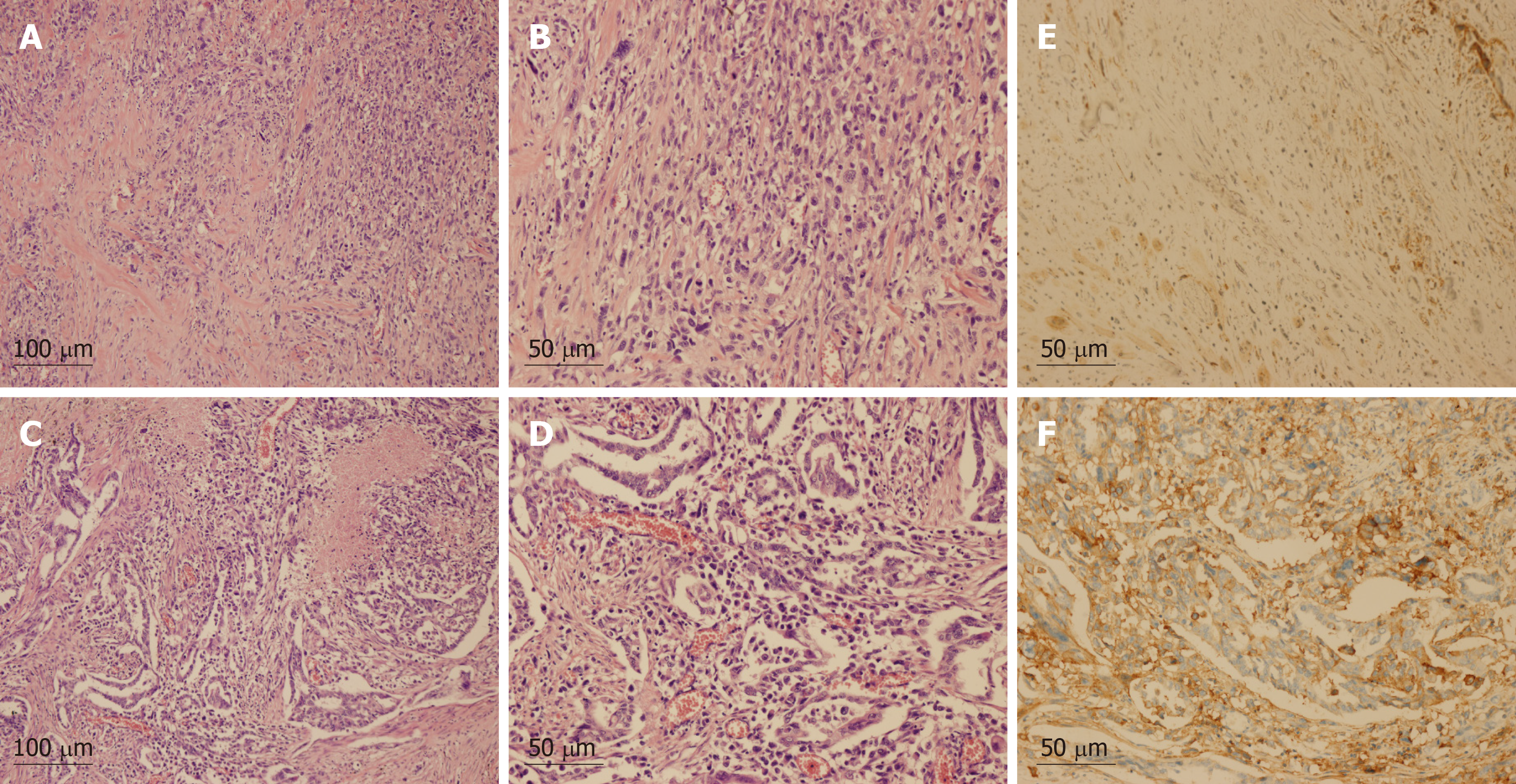
Pathology after right upper lobe pneumonectomy and lymphadenectomy revealed the following: centrally low differentiated carcinoma in the right upper lobe, (dominant with spindle cell/sarcomatoid carcinoma, adenocarcinoma also present), diameter of 63 mm, with pleura and chest wall invasion, pT3N0M0. Immunohistochemistry (IHC): PCK (+, partial cells), epithelial membrane antigen (+, partial cells), thyroid transcription factor 1 (+, for adenocarcinoma), Napsin A (+, for adenocarcinoma), smooth muscle actin (+, partial cells), p63 (-), cytokeratin K5/6 (-), S-100 (-), desmin (-), stabilin-2 (-). IHC for PD-L1 (clone SP142 by Ventana Medical System) showed strong expression in tumor cells (tumor proportion score [TPS]: 90%) (Figure 2). Next-generation sequencing analysis of the tumor sample revealed no mutation, rearrangement, or infusion. At 1 mo after surgery, CT revealed bilateral lung and adrenal gland metastases, and bone destruction was also found in the right second to third ribs and the left fifth rib, with soft tissue lesion formation in the right second to third ribs, representing stage IV (cT3N0M1b) PSC (Figure 1).
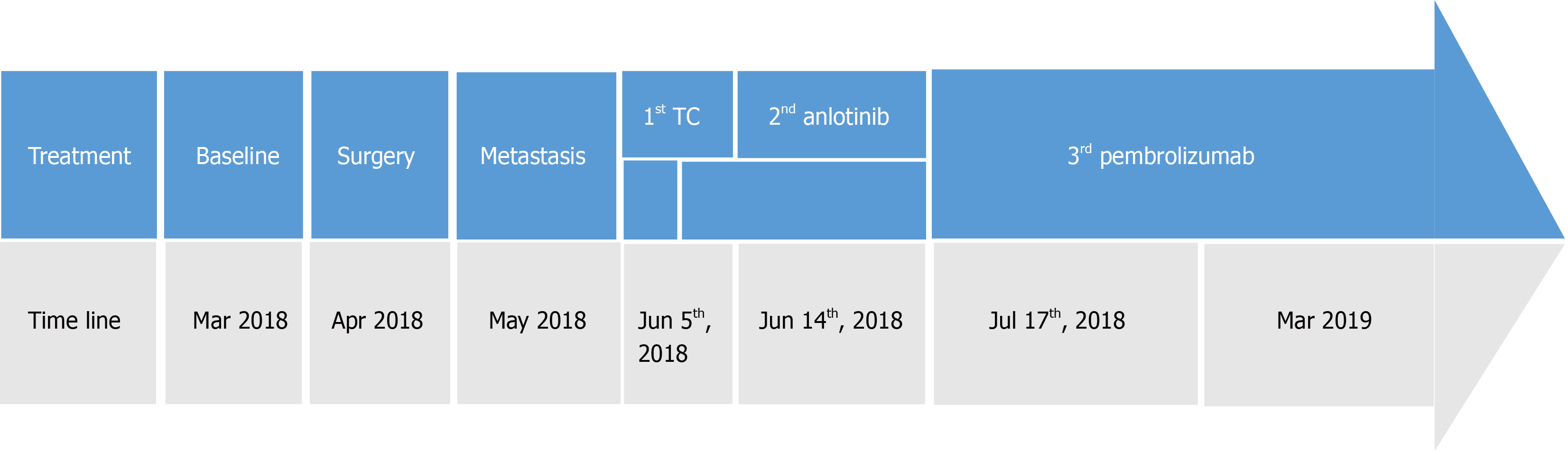
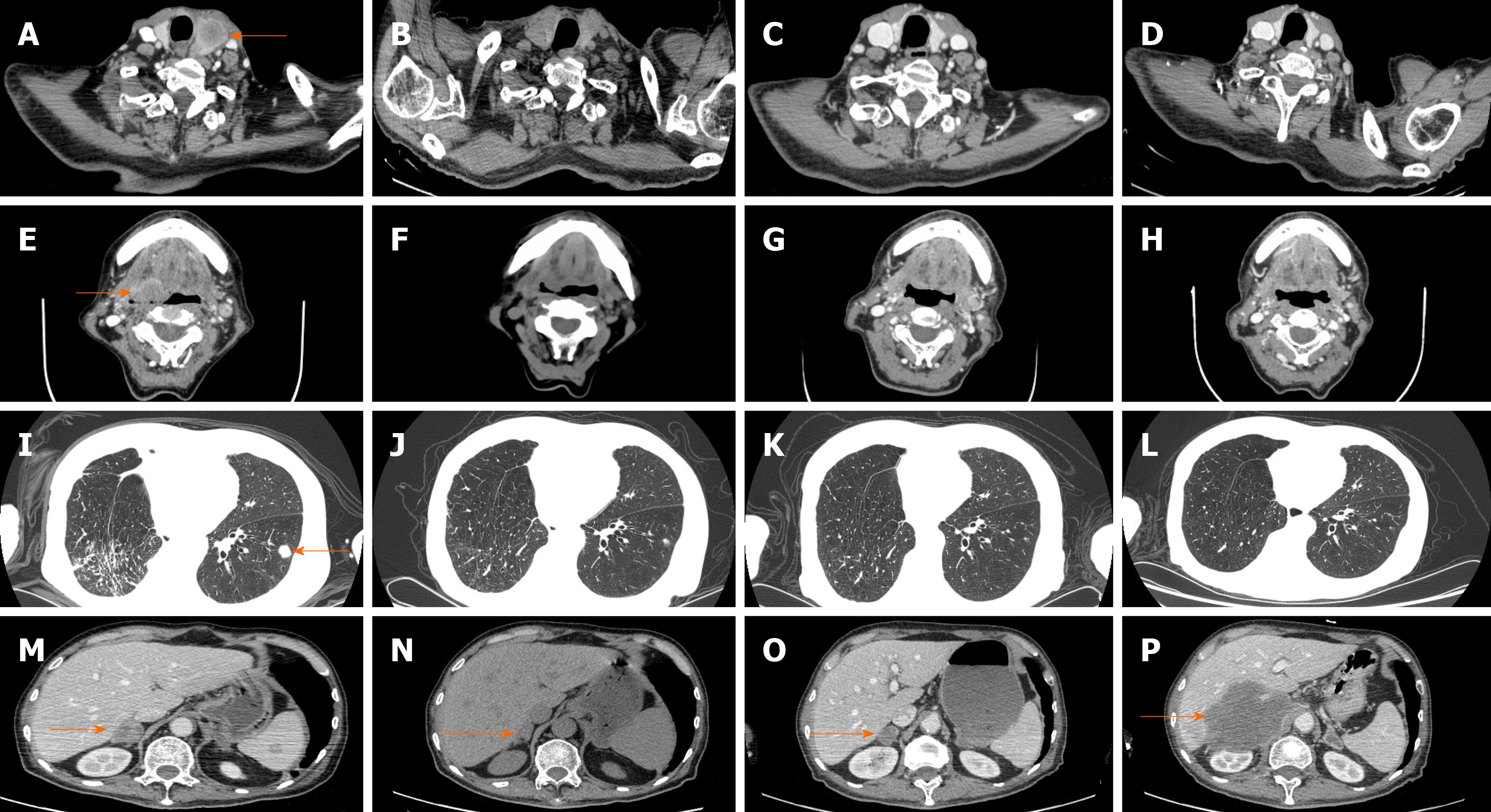
Systemic treatments were subsequently administered. On June 5, 2018, first-line chemotherapy was administered consisting of paclitaxel 220 mg and carboplatin 380 mg d1 q21d. At the same time, the patient complained of heavy pain in his right chest, with a numerical rating scale (NRS) score of 8, and oral morphine hydrochloride sustained-release tablets 30 mg q12h were prescribed. Starting on June 12, 2018, radiotherapy of the right chest wall was administered to relieve the pain, with 200 cGy each time. After chemotherapy, the patient experienced grade 4 leukopenia (0.8 × 109/L) and grade 2 nausea and vomiting according to the Common Terminology Criteria for Adverse Events version 4.0. Rapid enlargement of the left supraclavicular lymph nodes revealed progressive disease. Because of concerns that the patient could not tolerate toxic cell chemotherapy, second-line therapy was administered with the anti-angiogenic agent anlotinib 12 mg po qd1-14 q3w starting from June 14, 2018. Unfortunately, grade 3 hypertension with systolic blood pressure 160 mmHg, grade 2 nausea/vomiting, fatigue and loss of appetite was observed; therefore, anlotinib was discontinued on June 26, 2018. At the same time, radiation treatment also stopped, with the patient receiving a total of 1200 cGy. On June 30, 2018, the patient experienced cough with white sputum, white blood cell count: 14 × 109/L, C-reactive protein: 78.2 mg/L, and procalcitonin: 0.05 ng/mL, and auscultation revealed wet rales in both lungs. Consequently, the antibiotics piperacillin and sulbactam were administered, and the infection gradually resolved. However, the patient again developed disease progression to the thyroid, oropharynx left anterior wall, hypopharynx left wall, left chest wall, bilateral lungs, neck lymph nodes, ribs, and adrenal glands. His PS score was 2 at that time. Because the tumor progression was so rapid and high PD-L1 expression was observed in this patient, a combination of isocyclophosphamide and pembrolizumab was planned on July 11, 2019. The patient exhibited severe allergic reactions, including tightness, throat edema, and redness after mesna was injected, which was administered to prevent urinary tract toxicity of isocyclophosphamide; therefore, treatment was suspended. The patient gradually recovered, and 100 mg of the anti-PD1 agent pembrolizumab (Keytruda) was given on July 17, 2018 (Figure 3). At 2 d after administration, the patient experienced a fever of 39.8 °C with cough and sputum; blood culture revealed Staphylococcus aureus, and sputum culture showed Candida albicans. As the patient also experienced grade 2 respiratory insufficiency and loss of appetite, immunotherapy was stopped. After 6 wk of linezolid antibiotics and fluconazole antifungal treatment, the patient recovered. Gradually, the patient’s pain was relieved to NRS 0; no analgesic was needed, and his PS score recovered to 0. However, the patient refused to continue immunotherapy. On September 18, 2018, a controlled CT scan showed shrinkage of all the lung metastases, both sites of rib bone destruction and the thyroid and adrenal gland metastases, Accordingly, partial response (PR) was achieved for this patient. No late toxicity was observed.
On November 30, 2018, another CT scan showed significant regression of all metastases except the lesions in the right adrenal gland, which were slightly larger, and a continuous PR was assessed. Considering that the lesion in the right adrenal gland was becoming larger while no late immunotherapy toxicity was observed, we advised the patient to receive further treatment, such as puncture biopsy, local radiotherapy for his right adrenal gland lesion and pembrolizumab. However, the patient refused. The patient’s ECOG status significantly deteriorated starting from March 2019, and a subsequent CT scan reassessment (March 12, 2019) showed disappearance of the metastatic lesions in the neck lymph nodes, thyroid, oropharynx and hypopharynx; the lesions in the bilateral lungs and chest walls also displayed sustained reduction, but the lesion in his right adrenal gland had become much larger. We advised the patient to receive further treatment, but he refused. Finally, on April 24, 2019, the patient died from disease progression. The tumor changes are characterized in Figure 4. The patient did not receive a CT scan from March 2019 to April 2019. Third-line pembrolizumab resulted in a progression-free survival (PFS) of 8 mo and an overall survival (OS) of 9 mo for this patient.
PSC is a rare subtype of NSCLC that is not sensitive to conventional chemotherapy, and the prognosis is much poorer than that of other types of NSCLC[16,17]. Fortunately, immunotherapy is offering new treatment possibilities for this disease. PD-L1 overexpression is very common in PSC[18]. The mechanism of action of immunotherapy is a multiple-step process related to inflammation. Once inflammation accelerates, an extensive systemic inflammatory response may occur, and the efficacy may be sustained and thorough[19]. One study reported that a PSC patient treated with pembrolizumab achieved a PR[20]. Another PSC patient with small intestinal metastasis achieved a PR after 3 mo of immunotherapy. Due to pneumonia, immunotherapy was discontinued, and the disease was still well controlled during reexamination 7 mo later[21]. The present patient obtained 8 mo of control of most of his lesions after only one administration of pembrolizumab. This outcome shows that PSC is sensitive to immunotherapy.
Radiotherapy is widely used in limited disease with perfect local tumor control; for advanced disease, radiation is traditionally used for palliative purposes. Currently, increasing attention has been paid to the combination of radiotherapy and immunotherapy[22]. Tumor cell death after radiotherapy can release neoantigens[23,24], and anti–PD-1/PD-L1 therapy can augment the radiation-induced immune response, even causing an abscopal effect. In the PACIFIC study, patients in the durvalumab group had longer PFS and OS than those in the controlled group. Additionally, a secondary analysis of KEYNOTE- 001 revealed that PFS and OS were significantly longer for patients who had previous extracranial radiation before pembrolizumab than for patients who did not have previous radiation. Elevated PD-L1 expression may continue for 7 d at the end of radiotherapy; consequently, if PD-L1 inhibitors are used within this time, efficacy may be increased. Based on subgroup analysis in the PACIFIC study, a better PFS was observed when durvalumab was initiated within 2 wk of radiation compared with durvalumab > 2 wk after radiation. Previous studies have suggested that high ablative doses are highly effective; however, a high dose of radiotherapy may lead to lymphopenia and thereby reduce the effectiveness of immunotherapy. At present, researchers tend to believe that a lower dosage, such as 2000 cGy, is sufficient to achieve a sensitization effect. As we all known, PSC is a type of tumor which is not sensitive to radiotherapy. For this patient, he receives radiotherapy from June 12 to June 26, totally 1200 cGy to relieve chest wall pain, a CT scan between the end of radiotherapy and start of immunotherapy showed tumor progression. And after pembrolizumab, a controlled CT revealed that the tumor shrank obviously. So, the clearance of the tumor is probably due to immunotherapy or due to the combination of radiotherapy and immunotherapy.
In our case, the patient experienced different efficacies with regard to his metastases: most of the metastases were perfectly controlled, though the lesion in the right adrenal gland first shrank and then progressed. Intratumor heterogeneity (ITH) was believed to be present in this patient. Indeed, recent studies have revealed that ITH can exist within a single patient and even a single tumor. During “tumor progression,” some cancer cells may develop additional mutations, leading to the emergence of subclones; at the same time, cancer cells may lose functional T-cells and become dramatically different from the original cancer cells in response to treatment. These progression characteristics are collectively called “acquired resistance.” This polyclonality or ITH represents a major obstacle for the success of treatment. Adenocarcinoma is the most frequent histotype of PSC[25]. Sarcomatoid carcinoma and adenocarcinoma were both present in our patient, and the different pathology and biology likely indicate different efficacies for the same treatment. In theory, radiation of multiple lesions may not only reduce disease burden but also stimulate more robust and more diverse immune cells, leading to increased and diverse antigen stimulation, presentation and T-cell priming systemically, thereby abrogating resistance. Therefore, if the patient had undergone a biopsy of the right adrenal gland or received radiotherapy of the right adrenal gland and continued immunotherapy, he may have gained a longer period of tumor control.
The efficacy of pembrolizumab may be better for patients with high PD-L1 expression; at the same time, adverse events may also be severe. The present patient experienced fever with infection after therapy. Analysis of the KEYNOTE-001 trial showed that patients with previous thoracic radiotherapy were likely to have treatment-related pulmonary toxicity after pembrolizumab, but there were no significant differences in grade 3 or worse pulmonary toxicities. In the PACIFIC study, the addition of durvalumab significantly increased toxicity, with one-third of the patients discontinuing immunotherapy due to pneumonitis. As both lung radiotherapy and immunotherapy can lead to pneumonitis, toxicity should be carefully monitored in patients receiving such combined treatment.
To the best of our knowledge, this is the first report of pembrolizumab administration for metastatic PSC in which most of the lesions achieved a perfect response for 8 mo after only one dosage of only 100 mg. There are some notable characteristics of this patient. First, this was a 73-year-old patient. It is known that immunotherapy will be less effective for old patients than young patients, and old age may also be a factor for hyperprogression disease after immunotherapy. Second, the patient was at PS 2 when the immunotherapy was taken; most clinical trials will exclude patients with poor PS scores, and studies suggest that patients with poor PS scores experience less treatment efficacy. Third, PD-L1 was overexpressed in this patient, and he experienced severe adverse events. Some studies have shown that severe adverse events after immunotherapy are associated with better efficacy and longer periods of tumor control. Fourth, the patient received pembrolizumab only once at 21 d after radiotherapy, the dosage of which was 1200 cGy, and most of his lesions were perfectly controlled. It is believed that the radiotherapy plays a very important role in sustained tumor control; efficacy may be increased with a shorter interval between radiotherapy and immunotherapy. Fifth, the patient experienced different efficacies for his metastases, and ITH was believed to exist in this patient. A longer tumor control time may have occurred if the patient had received radiotherapy of the right adrenal gland and continued immunotherapy. This case provides valuable insights into the use of immunotherapy in PSC. First, the poor prognosis of this disease makes it necessary to search for new treatment options. Second, high PD-L1 expression enables deep and sustained remission with immunotherapy. Finally, radiotherapy followed by immunotherapy may be a promising choice to improve treatment efficacy. Further studies should be performed to explore the optimization of treatment for this disease. The finding also raises some questions about the appropriate usage of immunotherapy, namely, what is the appropriate dosage, how often should it be used, and is there some method to reduce adverse events. At present, it is recommended that PD-1 and PD-L1 inhibitors be administered every 2 or 3 wk, as it is known that, the terminal half-life of pembrolizumab is 26 d. It should be determined whether changing the immunotherapy schedule to a longer time is a safe, effective, and cost-saving strategy while reducing patients’ adverse events. It seems that a lower frequency or lower dosage may yield the same effectiveness, especially for patients with PD-L1 overexpression or when immunotherapy was followed after radiotherapy.
Nevertheless, there were some limitations. First, the PD-L1 test was used for SP142; Dako 22C3 is the standard diagnostic method for pembrolizumab, and SP142 is relatively lower in sensitivity. Regardless, the patient showed high expression by SP142, which may detect the true high expression of PD-L1. Second, PD-L1 expression was assessed using tissue obtained before chemotherapy and radiotherapy. Therefore, true PD-L1 expression after chemotherapy and radiotherapy was unclear. Moreover, the relationship between PD-L1 expression after chemotherapy and radiotherapy and the effectiveness of pembrolizumab are worthy of investigation. Third, when the right adrenal lesion progressed, rebiopsy was not performed; consequently, it is unknown whether there were differences in pathology or biology between lesions in the pulmonary and the right adrenal gland.
PSCs with PD-L1 overexpression may achieve a good response and survival benefit from pembrolizumab. The combination of radiotherapy and immunotherapy may be a promising treatment in PSC. More studies should be performed to obtain a deeper understanding of this disease and to find more optimized treatments.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tabll AA, Kamimura K S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Rahouma M, Kamel M, Narula N, Nasar A, Harrison S, Lee B, Stiles B, Altorki NK, Port JL. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg. 2018;53:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Ung M, Rouquette I, Filleron T, Taillandy K, Brouchet L, Bennouna J, Delord JP, Milia J, Mazières J. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer. 2016;17:391-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Roesel C, Terjung S, Weinreich G, Hager T, Chalvatzoulis E, Metzenmacher M, Welter S. Sarcomatoid carcinoma of the lung: a rare histological subtype of non-small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg. 2017;24:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Ouziane I, Boutayeb S, Mrabti H, Lalya I, Rimani M, Errihani H. Sarcomatoid carcinoma of the lung: a model of resistance of chemotherapy. N Am J Med Sci. 2014;6:342-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Vieira T, Girard N, Ung M, Monnet I, Cazes A, Bonnette P, Duruisseaux M, Mazieres J, Antoine M, Cadranel J, Wislez M. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8:1574-1577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5948] [Cited by in F6Publishing: 6767] [Article Influence: 845.9] [Reference Citation Analysis (0)] |
| 7. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3449] [Cited by in F6Publishing: 4128] [Article Influence: 688.0] [Reference Citation Analysis (0)] |
| 8. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4116] [Cited by in F6Publishing: 4712] [Article Influence: 589.0] [Reference Citation Analysis (0)] |
| 9. | Vieira T, Antoine M, Hamard C, Fallet V, Duruisseaux M, Rabbe N, Rodenas A, Cadranel J, Wislez M. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer. 2016;98:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Sim JK, Chung SM, Choi JH, Oh JY, Lee SH, Kim JH, Min KH, Hur GY, Shim JJ, Kang KH, Shin BK, Lee JH, Lee SY. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J Intern Med. 2018;33:737-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Kim S, Kim MY, Koh J, Go H, Lee DS, Jeon YK, Chung DH. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer. 2015;51:2698-2707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8:803-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Gounant V, Brosseau S, Naltet C, Opsomer MA, Antoine M, Danel C, Khalil A, Cadranel J, Zalcman G. Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer. 2016;99:162-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Salati M, Baldessari C, Calabrese F, Rossi G, Pettorelli E, Grizzi G, Dominici M, Barbieri F. Nivolumab-Induced Impressive Response of Refractory Pulmonary Sarcomatoid Carcinoma with Brain Metastasis. Case Rep Oncol. 2018;11:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Sukrithan V, Sandler J, Gucalp R, Gralla R, Halmos B. Immune Checkpoint Blockade Is Associated With Durable Responses in Pulmonary Sarcomatoid Carcinoma. Clin Lung Cancer. 2019;20:e242-e246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Maneenil K, Xue Z, Liu M, Boland J, Wu F, Stoddard SM, Molina J, Yang P. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer. 2018;19:e323-e333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Antoine M, Vieira T, Fallet V, Hamard C, Duruisseaux M, Cadranel J, Wislez M. [Pulmonary sarcomatoid carcinoma]. Ann Pathol. 2016;36:44-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Nakagomi T, Goto T, Hirotsu Y, Shikata D, Yokoyama Y, Higuchi R, Amemiya K, Okimoto K, Oyama T, Mochizuki H, Omata M. New therapeutic targets for pulmonary sarcomatoid carcinomas based on their genomic and phylogenetic profiles. Oncotarget. 2018;9:10635-10649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Alì G, Bruno R, Poma AM, Affinito O, Monticelli A, Piaggi P, Ricciardi S, Lucchi M, Melfi F, Chella A, Cocozza S, Fontanini G. Whole transcriptome targeted gene quantification provides new insights on pulmonary sarcomatoid carcinomas. Sci Rep. 2019;9:3536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Schrock AB, Li SD, Frampton GM, Suh J, Braun E, Mehra R, Buck SC, Bufill JA, Peled N, Karim NA, Hsieh KC, Doria M, Knost J, Chen R, Ou SI, Ross JS, Stephens PJ, Fishkin P, Miller VA, Ali SM, Halmos B, Liu JJ. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J Thorac Oncol. 2017;12:932-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Vieira T, Antoine M, Ruppert AM, Fallet V, Duruisseaux M, Giroux Leprieur E, Poulot V, Rabbe N, Sclick L, Beau-Faller M, Lacave R, Lavole A, Cadranel J, Wislez M. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer. 2014;85:276-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Turgeon GA, Weickhardt A, Azad AA, Solomon B, Siva S. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med J Aust. 2019;210:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1219] [Cited by in F6Publishing: 1475] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 24. | Yu WD, Sun G, Li J, Xu J, Wang X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019;452:66-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 25. | Kaira K, Horie Y, Ayabe E, Murakami H, Takahashi T, Tsuya A, Nakamura Y, Naito T, Endo M, Kondo H, Nakajima T, Yamamoto N. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol. 2010;5:460-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |