Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2870
Peer-review started: March 2, 2020
First decision: April 7, 2020
Revised: May 7, 2020
Accepted: June 13, 2020
Article in press: June 13, 2020
Published online: July 6, 2020
Patients undergoing liver transplantation can develop posterior reversible encephalopathy syndrome (PRES) and acute heart failure (HF) in the post-operative period. But PRES with HF caused by tacrolimus has rarely been described.
A 40-year-old female patient who had a normal preoperative cardiac and neural evaluation developed PRES with acute heart failure tacrolimus-induced after liver transplantation. The challenges associated with both diagnosis and management in the setting of a newly implanted graft are discussed.
Tacrolimus can induce neurotoxicity and then cardiac toxicity. Magnetic resonance imaging, echocardiography, and increased brain natriuretic peptide may be predictive of post-operative PRES with acute heart failure. Further investigations are necessary to verify this finding.
Core tip: We describe a case of tacrolimus-induced posterior reversible encephalopathy syndrome with acute heart failure, which developed after liver transplantation in a patient who had a normal preoperative cardiac evaluation.
- Citation: Liu JF, Shen T, Zhang YT. Posterior reversible encephalopathy syndrome and heart failure tacrolimus-induced after liver transplantation: A case report. World J Clin Cases 2020; 8(13): 2870-2875
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2870.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2870
Liver transplantation (LT) has become an effective therapeutic method for end-stage liver disease. Calcineurin inhibitors revolutionized the management of patients who underwent organ transplantation by effectively reducing acute rejection episodes and improving survival[1]. However, neurotoxicity and nephrotoxicity are the major adverse effects. Posterior reversible encephalopathy syndrome (PRES) is the most severe and dramatic consequence of calcineurin inhibitor neurotoxicity. Patients typically present with altered mental status, headache, vomiting, focal neurological deficits, visual disturbances, and seizures. Magnetic resonance imaging (MRI) is the most sensitive imaging test for detection. PRES is well accepted as a neuroradiological phenomenon diagnosed by MRI in the liver, kidney, hematopoietic stem cells and heart transplant patients. Heart failure (HF) is an important potential complication in liver transplant patients, but PRES with HF caused by tacrolimus has rarely been described. Here, we report one such case that was successfully identified and managed appropriately.
A 40-year-old female patient presented with psychiatric symptoms of acute confusional state and tonic-clonic seizures at the early stage after LT.
The patient was admitted to our hospital with complaints of repeated abdominal distension and jaundice for more than 10 mo. She was diagnosed with primary biliary cholangitis. The complications after primary diagnosis included esophageal gastric varices, ascites, and splenomegaly. She had a model for end-stage liver disease score of 20. Electrocardiogram (known as ECG) demonstrated normal sinus rhythm with QTc of 420 ms. An echocardiogram indicated normal cardiac function with an ejection fraction (EF) of 66%.
She successfully underwent an uneventful modified piggyback LT on August 26, 2017 and was hemodynamically stable throughout surgery. Her previous anti-rejection (immunosuppressive) therapy after LT consisted of mycophenolate mofetil, methylprednisolone, and tacrolimus. We reduced the dosage of corticosteroids gradually and paid special attention to her fluid and electrolyte balance. The graft function was excellent. At 7 d after the transplantation and 3 d after tacrolimus (Prograf 0.5 mg; Astellas Pharma China Inc., Kerry, Ireland) treatment (1 mg/d), she developed psychiatric symptoms of acute confusional state with a mean arterial pressure (MAP) of about 80 mmHg. A psychiatrist and neurologist were consulted. Quetiapine tablets were given to control her mental symptoms. Furthermore, she presented with tonic-clonic seizures on day 10 after LT and on day 6 of tacrolimus treatment. She did not complain of chest pain and dyspnea.
The patient had no history of prior neurological complications, hypertension and coronary artery disease or other cardiac abnormalities.
Vital signs were elevated systolic blood pressure of 170/90 mmHg (cuff blood pressure), MAP of about 110 mmHg, increased heart rate of 120 per min, and a small amount of moist rales in both lower lungs. No focal neurological deficits were noted on physical examination.
Tacrolimus blood level was 4.10 ng/mL, serum brain natriuretic peptide (known as BNP) level was > 5000 pg/mL (normal < 89 pg/mL), and troponin I (known as TNI) level was 0.032 pg/mL (normal < 0.016 ng/mL).
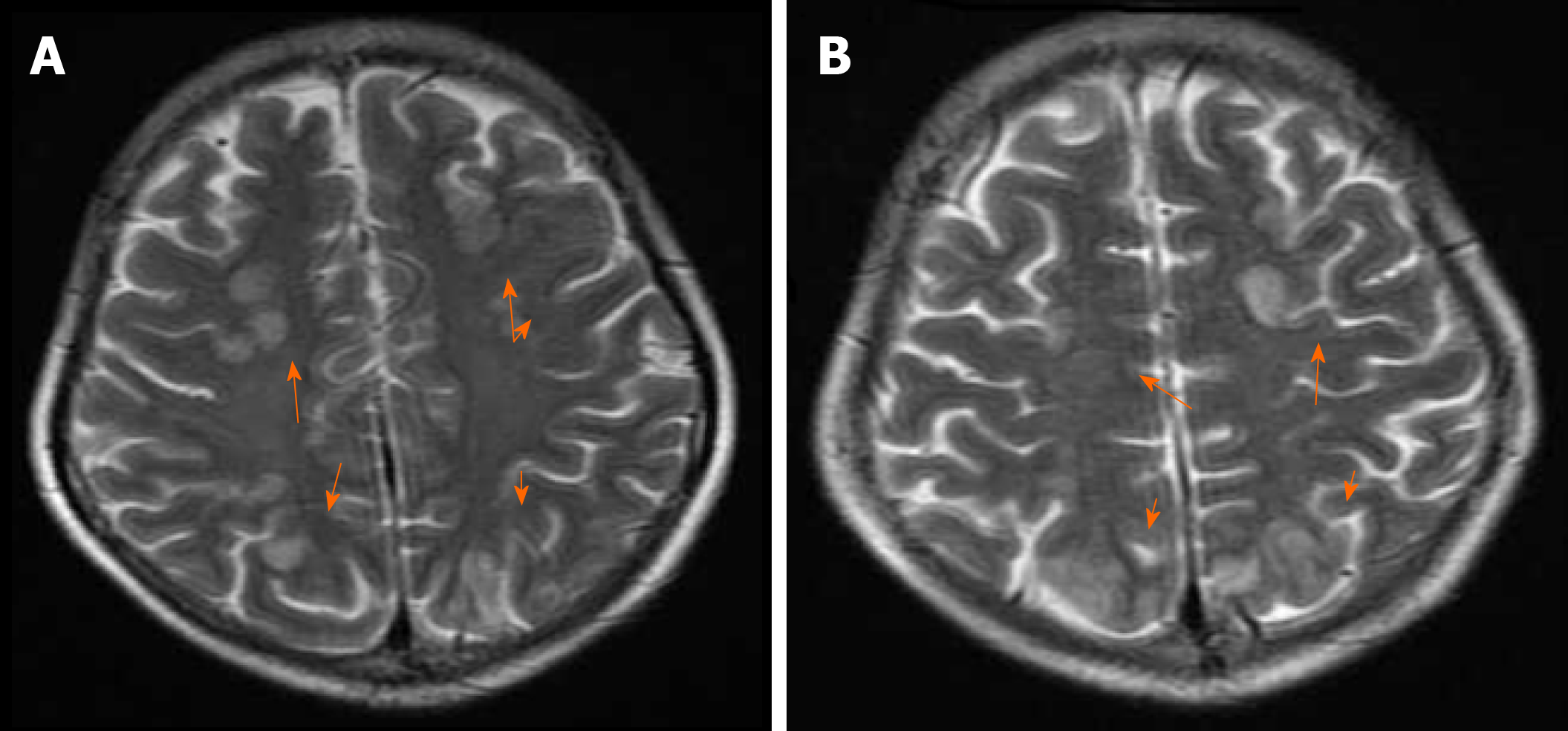
ECG demonstrated sinus tachycardia with no ST-T changes. Brain MRI was performed and showed a cerebral cortex subcortical FLAIR hyperintensity area in the bilateral frontal, parietal, and occipital lobes, which was consistent with PRES associated with tacrolimus (Figure 1). Considering her MRI findings and recent use of tacrolimus, PRES was initially diagnosed. Meanwhile, an echocardiogram indicated diffuse hypokinesia of the left ventricle (LV) with an EF of 40% compared with 66% before and 60.7% 4 d after surgery, respectively.
The final diagnosis of the presented case was PRES and HF tacrolimus-induced after LT.
Tacrolimus was terminated after final diagnosis. Sodium valproate and levetiracetam tablets were given to prevent epilepsy. Urapidil was administered to control her blood pressure. Cardiological treatment with isosorbide mononitrate and furosemide was administered.
The patient showed improvement in mental status and motor symptoms. Her serum BNP and TNI levels decreased. A subsequent electroencephalogram showed mild-to-moderate abnormalities without clear epileptiform discharges. Within 1 wk, the patient regained normal mental status with no sensory or motor deficits. Immunosuppressive treatment was switched to mycophenolate mofetil and cyclosporine (100 mg/d) reaching a blood level of 90 ng/mL. Laboratory work-up revealed stable graft function (serum albumin and transaminases were normal, serum total bilirubin was < 40 μmol/L).
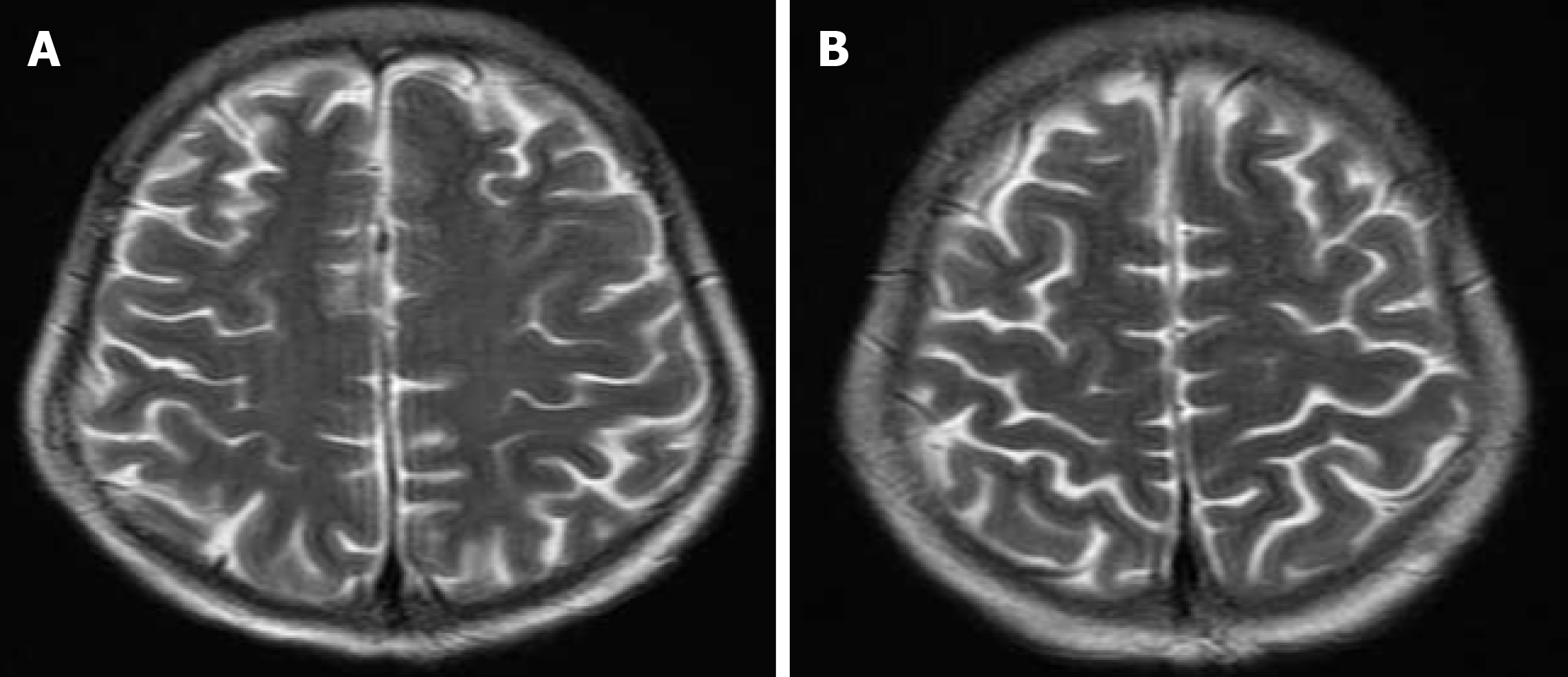
A follow-up MRI after 4 wk demonstrated resolution of previous FLAIR signal abnormalities, with no abnormal post-contrast enhancement, confirming the diagnosis of PRES (Figure 2). No further episodes of psychotic and seizures were reported after the discontinuation of tacrolimus and levetiracetam. A follow-up echocardiogram showed only mild regurgitation of mitral and tricuspid valves. LV systolic function returned to normal (EF 55%). The graft function remained stable and at 31 mo after transplantation, the patient is enjoying good general condition and good graft function.
The pathophysiological mechanism underlying PRES is not fully understood. Postulated hypotheses include medication-induced endothelial damage and hyperperfusion due to disruption of the cerebral autoregulation system. Endothelial dysfunction is a key factor[2]. Transient vasogenic edema can be detected on MRI. The cause of neurotoxicity with PRES remains controversial. Frequently implicated triggers are eclampsia, hypertensive emergency, or exposure to immunosuppressive therapy. PRES is also reportedly associated with infection, sepsis, shock, autoimmune disease, and chemotherapy[3]. Calcineurin inhibitors are commonly used immunosuppressants. Medication-induced PRES is the most frequently reported adverse event, occurring soon after transplantation (averaging 31 d)[3]. Our patient developed PRES on day 10 after transplantation. The widespread use of tacrolimus inadvertently resulted in an escalating incidence of neurotoxic adverse effects. Tacrolimus reduces the expression of p-glycoprotein in the brain endothelium, leading to dysfunction of the blood-brain barrier and vasogenic edema, which most likely result in changes in MRI-detectable intensity of various brain regions[4]. Previous studies have indicated that polymorphisms in drug-metabolizing genes can explain the propensity towards neurotoxicity. Tacrolimus-related PRES is unrelated to the drug levels. Tacrolimus-related PRES can even occur in transplant patients as early as the first week of initiation of anti-rejection therapy and needs to be promptly recognized after transplantation as delayed diagnosis can cause cytotoxic edema and result in permanent neurological sequelae. MR imaging with diffusion-weighted sequences provides not only a powerful means of diagnosing PRES but also a wealth of prognostic information about the patient[5]. When tacrolimus-related PRES occurs, immunosuppressive therapy may be safely and efficiently switched to cyclosporine, everolimus, sirolimus, and other immunosuppressants.
The cause of HF in the early post-operative period may be multifactorial. High-quality graft was used for the present case and the surgical procedure was, in general, uncomplicated. There was no significant intraoperative hemodynamic instability. Her echocardiography did not indicate structural and contractile abnormalities and morphological changes, such as enlargement or hypertrophy of cardiac chambers before and within 1 wk after surgery. QT interval prolongation is a supportive criterion for the diagnosis of cirrhotic cardiomyopathy. Our patient’s QTc interval was 0.42 s which was in normal range (women and children ≤ 0.45 s). Because the newly diagnosed HF happened at the same time as PRES, drug induction was a likely explanation. The description of tacrolimus-induced cardiac toxicity in the literature is limited. Igata et al[6] reported a case of acute right HF caused by tacrolimus after renal transplantation. Their report by echocardiography indicated that the tacrolimus could induce the myocardial impairment in the setting of acute renal failure with elevated levels of tacrolimus. Thiru et al[7] studied the pathologic effects of tacrolimus in canine and baboon renal allograft recipients and found tacrolimus-induced vasculitis in the organs of these animals and thus suggested that vasculitis may be responsible for the cardiac toxicity from tacrolimus. Based on our case, we supposed that the tacrolimus could induce the myocardial impairment and subsequent myocardial failure.
The cardiotoxic effect of tacrolimus was primarily described in pediatric transplant recipients. Tacrolimus-induced cardiomyopathy is known with reduced left ventricular EF and left ventricular hypertrophy. The pathogenesis of tacrolimus-induced cardiomyopathy may be secondary to the binding of tacrolimus to FK506 binding proteins in cardiac muscle[8]. However, most of the cases showed cardiac abnormalities only following a significantly longer time of exposure to tacrolimus.
Takotsubo cardiomyopathy may be another likely etiology that should be considered. Takotsubo cardiomyopathy is a temporary heart condition that commonly occurs immediately after experiencing extreme emotional or physical stress. It has the same symptoms as a heart attack with chest pain and/or dyspnea, ST-T wave changes on ECG, and LV or biventricular dysfunction on imaging but is not caused by any underlying cardiovascular disease and resolves within a few days to several weeks. It is also known as stress cardiomyopathy, apical ballooning, or broken heart syndrome. It is usually diagnosed when invasive cardiac catheterization demonstrates unobstructed coronary arteries, and ventriculography shows characteristic LV ballooning leading to various degrees of acute LV dysfunction[9]. The occurrence of this disease is attributed to the large-scale production of catecholamines that causes myocardial hypokinesia via direct cardiomyocyte toxicity and induction of coronary microvascular dysfunction[10]. Takotsubo cardiomyopathy is described in particular in the presence of acute central nervous system pathologies. From this case, with reversal of PRES, the patient’s cardiac function normalized, perhaps suggesting that HF is secondary to PRES. However, this patient had no chest pain and dyspnea, and her echocardiogram indicated diffuse hypokinesia of LV rather than typical local apical ballooning and hypokinesia with preserved basal myocardial contractility. One limitation of the study is that we did not perform cardiac MRI or angiography in the patient to further verify this disease.
In conclusion, PRES with HF caused by tacrolimus has rarely been described. Our case demonstrates tacrolimus-induced neurotoxicity and cardiac toxicity (direct or indirect) and illustrates the need for heightened vigilance for neurological and cardiac complications after transplantation in patients receiving tacrolimus-based immunosuppression. It is very important for all physicians working in critical care to be aware of this entity which can be demonstrated by the follow-up MRI, echocardiography, and increased BNP. Early diagnosis followed by discontinuation of the causative agent, switching immunosuppressant regimen can result in resolution of symptoms. The etiology of PRES and acute HF after LT may be related to features of complex and unstable post-transplantation state. Further investigations are necessary to evaluate these findings.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Auzinger G, Kayaalp C S-Editor: Wang JL L-Editor: Filipodia E-Editor: Xing YX
| 1. | Song T, Rao Z, Tan Q, Qiu Y, Liu J, Huang Z, Wang X, Lin T. Calcineurin Inhibitors Associated Posterior Reversible Encephalopathy Syndrome in Solid Organ Transplantation: Report of 2 Cases and Literature Review. Medicine (Baltimore). 2016;95:e3173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 655] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 3. | Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 2008;29:924-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Wijdicks EF. Neurotoxicity of immunosuppressive drugs. Liver Transpl. 2001;7:937-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038-1048. [PubMed] [Cited in This Article: ] |
| 6. | Igata S, Wettersten N, Wong DJ, Sabet A, DeMaria AN. Acute right heart failure caused by tacrolimus after renal transplantation: Serial observation by speckle tracking and Doppler echocardiography. Echocardiography. 2017;34:1730-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Thiru S, Collier DS, Calne R. Pathological studies in canine and baboon renal allograft recipients immunosuppressed with FK-506. Transplant Proc. 1987;19:98-99. [PubMed] [Cited in This Article: ] |
| 8. | Bowman LJ, Brennan DC, Delos-Santos R, LaRue SJ, Anwar S, Klein CL. Tacrolimus-Induced Cardiomyopathy in an Adult Renal Transplant Recipient. Pharmacotherapy. 2015;35:1109-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018;104:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 151] [Article Influence: 15.1] [Reference Citation Analysis (2)] |