Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2679
Peer-review started: March 22, 2020
First decision: April 22, 2020
Revised: May 28, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 6, 2020
Chronic thromboembolic pulmonary hypertension (CTEPH) is a complex chronic disease in which pulmonary artery stenosis or obstruction caused by organized thrombus can lead to increased pulmonary artery pressure and pulmonary vascular resistance, ultimately triggering progressive right heart failure and death. Currently, its exact mechanism is not fully understood. Pulmonary endarterectomy (PEA) has immediate effects with low perioperative mortality and satisfactory prognosis in experienced expert centers for CTEPH patients with proximal lesions. Nevertheless, 37% of patients are deemed unsuitable for PEA surgery due to comorbidities and other factors, and nearly half of the operated patients have residual or recurrent pulmonary hypertension. Riociguat is the only approved drug for CTEPH, although its effect is limited. Balloon pulmonary angioplasty (BPA) is a promising alternative treatment for patients with CTEPH. After more than 30 years of development and refinements, emerging evidence has confirmed its role in patients with inoperable CTEPH or residual/recurrent pulmonary hypertension, with acceptable complications and comparable long-term prognosis to PEA. This review summarizes the pathophysiology of CTEPH, BPA history and development, therapeutic principles, indications and contraindications, interventional procedures, imaging modalities, efficacy and prognosis, complications and management, bridging and hybrid therapies, ongoing clinical trials and future prospects.
Core tip: Chronic thromboembolic pulmonary hypertension (CTEPH) is a serious chronic disease with a poor prognosis if left untreated. Pulmonary endarterectomy (PEA) is the only curable treatment of choice for certain CTEPH patients with proximal vessel lesions. Balloon pulmonary angioplasty (BPA) is a promising alternative interventional option for patients with CTEPH, especially those with distal lesions or residual/recurrent pulmonary hypertension after PEA. Herein, we summarize the pathophysiology of CTEPH, the history and development of BPA, therapeutic principles, indications and contraindications, interventional procedures, imaging modalities, efficacy and prognosis, complications and management, bridging and hybrid therapies, ongoing clinical trials and future prospects.
- Citation: Jin Q, Zhao ZH, Luo Q, Zhao Q, Yan L, Zhang Y, Li X, Yang T, Zeng QX, Xiong CM, Liu ZH. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: State of the art. World J Clin Cases 2020; 8(13): 2679-2702
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2679.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2679
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by precapillary pulmonary hypertension (PH) hemodynamically defined as mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg and a normal pulmonary artery wedge pressure ≤ 15 mmHg during resting right heart catheterization (RHC), with thromboembolic evidence showing mismatched perfusion defects in pulmonary arteries on computed tomographic pulmonary angiography (CTPA), lung perfusion scintigraphy or pulmonary angiography despite effective anticoagulation therapy for at least three months. If left untreated, CTEPH will rapidly progress and seriously threaten people’s lives[1], and CTEPH patients with mPAP > 30 mmHg have a relatively poor prognosis[2], with a 5-year survival rate of 30% for those with mPAP > 40 mmHg and 10% for those with mPAP > 50 mmHg[3,4].
Pulmonary endarterectomy (PEA) remains the potentially curative treatment of choice with an in-hospital mortality rate of 2.2% for all eligible CTEPH patients at experienced centers and should be considered whenever possible[5]. In most CTEPH patients, their postoperative hemodynamics return to normal or near normal, yet due to surgical inaccessibility and severe comorbidities, only around 63% are eligible for PEA, and 17%-31% of CTEPH patients experience persistent or recurrent PH after surgery[6-8], likely as a result of residual thrombi, concomitant pulmonary micro-vasculopathy, and recurrent thrombotic lesions[9,10]. In addition, some patients do decline invasive thoracotomy, which requires a meticulous technique and can only be performed by an interdisciplinary team at limited expert centers worldwide.
Currently, inoperable candidates have more options to receive medical and/or interventional therapies at specialized centers. With the great advances in pulmonary arterial hypertension (PAH) targeted drugs, including endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, soluble guanylate cyclase stimulators, prostacyclin and its analogues, and selective prostacyclin receptor agonists, the clinical condition and hemodynamic status among symptomatic PAH patients have been dramatically improved in recent years[11]; however, high-level evidence on the efficacy and safety of PAH targeted therapy for CTEPH patients remains far from enough worldwide[12-16]. Riociguat, a stimulator of soluble guanylate cyclase, is the first and only officially approved drug with an indication for inoperable or persistent/recurrent CTEPH. Nevertheless, it has limited effects on reducing mPAP despite improvements in pulmonary vascular resistance (PVR) and exercise tolerance[12]. Riociguat was marketed in China in June 2018 and was partially covered by the national health insurance in November 2019. Even so, most PH patients cannot benefit from this promising drug due to high costs.
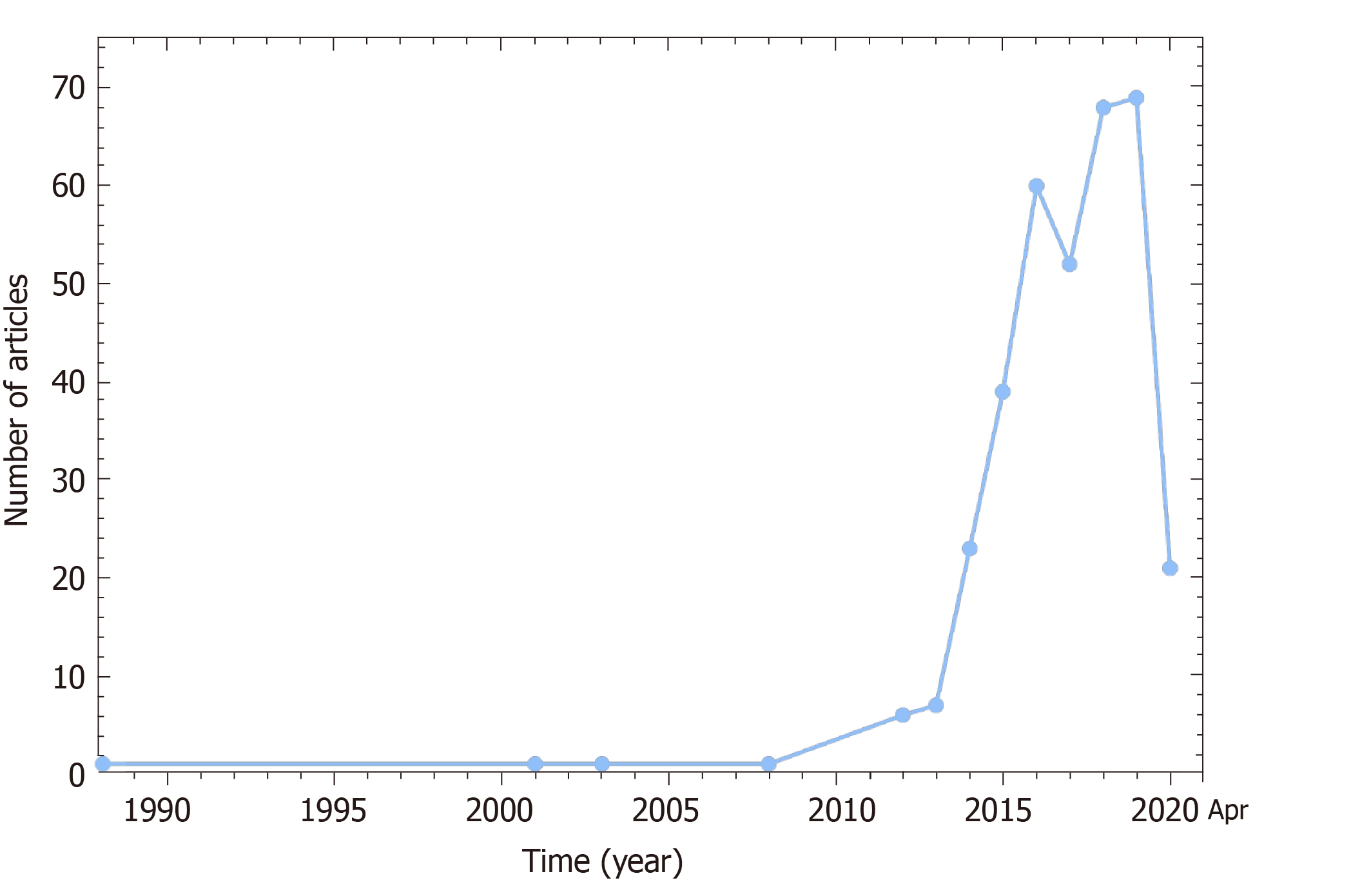
Balloon pulmonary angioplasty (BPA), also known as percutaneous transluminal pulmonary angioplasty, is a catheter-based interventional technique which uses appropriately sized balloons to dilate and open up stenotic or occluded pulmonary vessels under angiography guidance. Accumulating evidence suggests that it can strikingly improve hemodynamics, cardiac function and exercise tolerance with an acceptable incidence of complications, and the long-term prognosis is encouragingly comparable to PEA surgery[17,18]. After more than 30 years of development and refinements (Figure 1), BPA is emerging as a promising alternative treatment option for CTEPH patients. However, there are still many issues worth exploring for BPA, such as the selection of patients, the establishment of a standardized operation process and the reduction of complications, and this review aims to elaborate the latest progress of BPA from multiple perspectives.
Pathologic examinations disclosed massive hypertensive lesions in small pulmonary arteries characterized by microvascular remodeling, irregular intimal thickening and fibromuscular hyperplasia, eccentric intimal fibrosis, and plexiform lesions that shared some similarities to those seen in idiopathic PAH[19-21], likely as a result of endothelial cell proliferation/apoptosis dysregulation, endothelial to mesenchymal transition, abnormal proliferation and migration of fibroblasts and smooth muscle cells, and inflammatory cells infiltration[22,23]. Our previous proteomic analyses of endarterectomized tissues from CTEPH patients revealed that the detected proteins were mainly involved in immune and defense responses, inflammatory responses, and the complement and coagulation cascade[24]. The exact mechanisms of CTEPH remain poorly understood, genetic susceptibility and epigenetic modification, risk factors for thrombosis, imbalanced coagulation and fibrinolysis, inflammation and immune dysfunction, impaired angiogenesis, and vascular remodeling might collectively contribute to the onset and progression of CTEPH[19,20,25,26].
Unresolved organized thromboembolic material triggers narrowing or obliteration of pulmonary arteries, resulting in secondary redistribution of pulmonary blood flow between occluded and non-occluded vessels. Hemodynamic changes such as increased intravascular pressures and endothelial shear stress trigger microvascular endothelial dysfunction and vascular remodeling of pulmonary vasculature[8]. Increased vascular resistance causes elevated pulmonary arterial pressure, which ultimately leads to increased right heart afterload and even right heart failure. As the tension of the right ventricular (RV) wall increases, the interventricular septum shifts to the left, affecting left ventricle filling and cardiac output. In addition, organized thrombi block the blood flow path through the lungs, resulting in less blood entering the lungs for oxygenation and a severe mismatch of ventilation and perfusion, which in turn results in hypoxemia[27].
Balloon angioplasty was firstly described to treat peripheral pulmonary artery stenosis in 1980[28]. Soon afterwards, Lock et al[29] surgically created experimental bilateral branch pulmonary artery stenosis in newborn lambs, and validated the feasibility of balloon dilation in relieving acquired pulmonary artery narrowing, which directly impelled their initial clinical extension of balloon dilation angioplasty to similar lesions. Later in 1983, they demonstrated balloon dilation angioplasty could substantially decrease RV pressure and gradient across the lesion, and increase pulmonary blood flow among children with either hypoplastic or stenotic pulmonary arteries[30]. Since then, several studies focusing on the successful use of balloon angioplasty in peripheral pulmonary stenosis were reported[31-38], indicating the highly reasonable therapeutic possibility in pulmonary vascular diseases.
Balloon angioplasty was initially attempted in 1988 to treat acute pulmonary thromboembolism in the right descending branch of the pulmonary artery in a patient who had poor response to anticoagulant and thrombolytic therapy[39], but for CTEPH patients, the first BPA case was published in the same year by Voorburg et al[40]. In 2001, Feinstein et al[41] described the first case series certifying remarkable improvements in pulmonary hemodynamics, cardiac function and exercise tolerance in eighteen CTEPH patients treated with BPA. However, eleven patients developed reperfusion pulmonary edema (RPE), three patients required mechanical ventilation, one died of right heart failure 1 wk after BPA, and one died of aspiration pneumonia after 16 mo of follow-up. Due to potentially lethal complications, high mortality rates and non-superiority to PEA, BPA was not widely adopted and used for CTEPH patients. The progress of this interventional technique was almost stagnant for more than a decade, with only sporadic cases reported during this period[42-44].
In 2012, three reports from Japanese centers reignited the spark for the booming development of BPA[45-47], Japanese investigators have refined the BPA technique and established safer and more effective strategies by using smaller balloons and intravascular imaging, and by limiting the number of vessel segments per session. Compared to the initial report from 2001, BPA has been reported to provide encouraging improvements in hemodynamics, RV function and exercise capacity, with lower rates of fatal complications. After years of unremitting efforts, the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines recommended that BPA only be performed in experienced CTEPH expert center where medical therapy could be concurrently initiated[48,49]. PAH targeted therapy with or without BPA has been proposed in the newly updated treatment algorithm for inoperable CTEPH patients[50], and BPA is now enthusiastically pursued by numerous hospitals and institutions around the world.
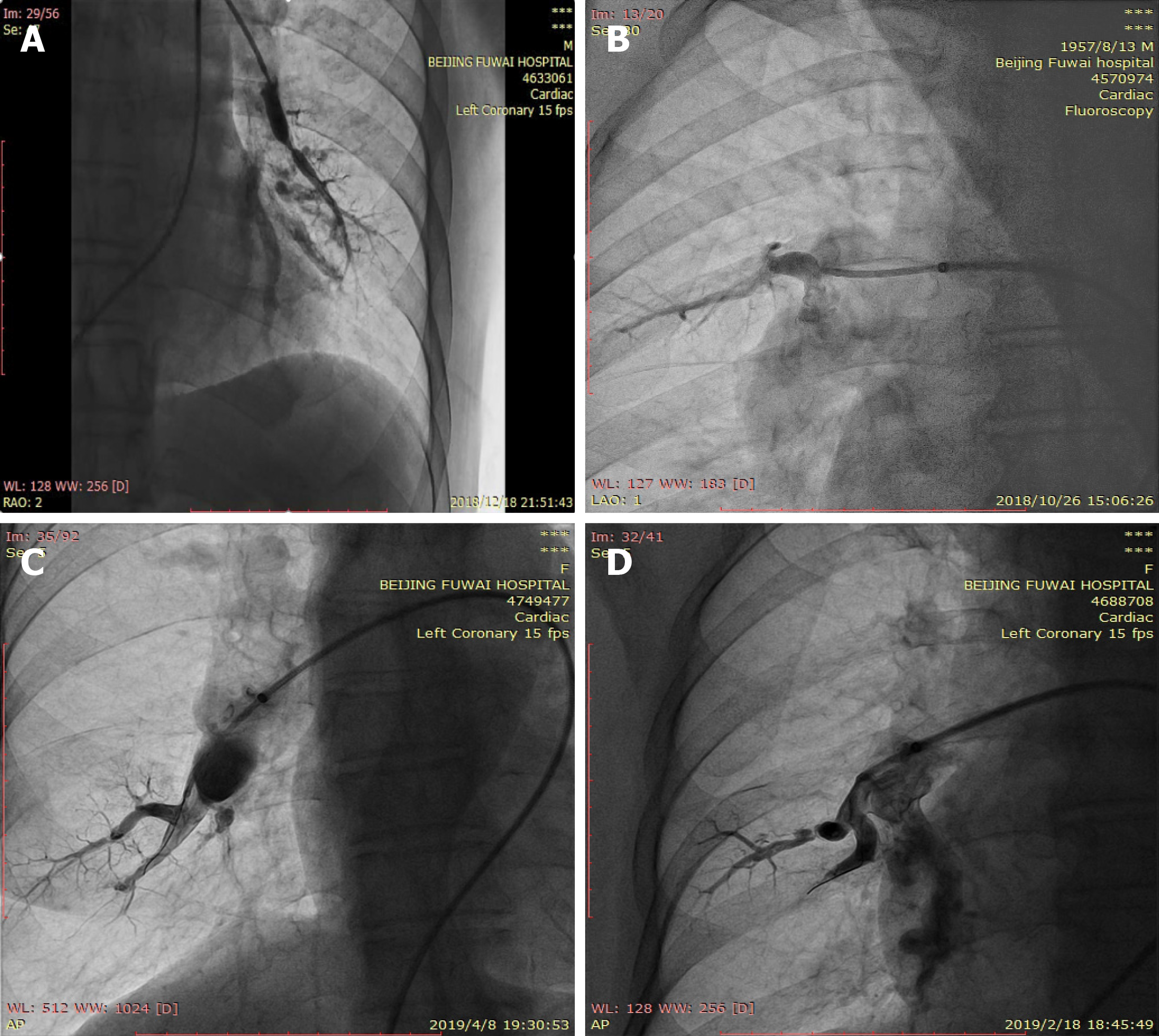
PEA immediately lowers and even normalizes pulmonary vascular hemodynamics by removing the accessible organized thrombus in culprit pulmonary arteries within one procedure (Figure 2). Nevertheless, BPA cannot handle most of the lesions at once due to the highly possible occurrence of lethal complications such as RPE and vascular injuries. Staged BPA ameliorated hemodynamics by eliminating stenosis and obstruction in the peripheral pulmonary arteries without physically removing intravascular organized thrombi. However, how stenotic or occlusive pulmonary arteries are enlarged and how hemodynamics are improved by BPA remain poorly explored[51-53].
Pathological findings of the dilated lesions revealed that dissections occurred in the medial vessel wall near the internal lamina elastica after BPA which exactly resembled what the expert surgeons performed during PEA, and the organized clots were compressed toward one side, shaping an enlarged pseudo-vascular space on the inner surface of which new intima was formed. It was speculated that web lesions were treated by dissection in the media and compression of existed microchannels, while band lesions were dilated through enlargement of a preexisting lumen without dissection. Dissection and compression of organized thrombi enlarged the vessel lumen, increased blood flow, and eventually improved pulmonary vascular hemodynamics[51,52]. Optical coherence tomography (OCT) also confirmed that the obstructive material was destroyed and eccentrically compressed to vessel walls immediately after BPA[47,54,55]. Intravenous ultrasound (IVUS) images indicated the immediate enlargement of the vessel lumen after BPA was mainly due to overall vessel stretching and slight compression of fibrous thrombus. The amount of fibrous tissue varied among different vessel lesion types, with the least fibrous tissue in band lesions, the most in subtotal occlusion lesions and intermediate in web lesions. The enlarged lumen area of the band-shaped lesion was caused by arterial wall stretching; however, in addition to vascular stretching, the web lesion was also compressed to one side of the vascular lumen, forming new microchannels. The mechanism of lumen enlargement at the site of subtotal occlusion was similar to that of the web lesion, but the organized thrombus was only mildly compressed owing to a large amount of fibrous tissues, indicating limited efficacy might be achieved in lesions rich in fibrous tissue[53,56].
The conventional University of California, San Diego selection criteria for PEA include: (1) World Health Organization functional class (WHO-FC) III or IV; (2) mPAP ≥ 30 mmHg and PVR ≥ 3.75 Wood units; (3) Surgically accessible thrombus in the main, lobar or proximal segmental pulmonary arteries; (4) No severe comorbidities; and (5) Patients who understand possible risks or complications, and signed written informed consent before procedures[57,58]. PEA indications have been gradually broadened during these years, and the 2015 ESC/ERS general criteria include: (1) Preoperative WHO-FC II-IV; and (2) Surgically accessible lesions located in the main, lobar or segmental pulmonary arteries[48].
BPA indications include: (1) Ineligible for PEA (surgically accessible but inoperable CTEPH due to comorbidities, inoperable CTEPH with inaccessible lesions, residual or recurrent PH after PEA); (2) Symptomatic CTEPH with WHO-FC III-IV despite optimal medication; (3) Without serious complications, multi-organ failure, or iodine allergy; and (4) Individuals who understand the purpose, process, risks and benefits, alternative options and voluntarily give informed consent[59,60]. Theoretically, BPA treatment can be considered as long as mPAP > 25 mmHg with angiographically accessible lesions[61]. The effects of BPA on inoperable CTEPH with surgically inaccessible lesions and residual/recurrent PH after PEA have been well documented[62,63], yet the roles of BPA for operable CTEPH with technically accessible lesions but deemed unsuitable for surgery (due to comorbidities, subjective self-determination or informed refusal) and chronic thromboembolic disease (CTED) have not been well established and need further exploration and observation[64-66]. If surgical PEA is not possible for central-type lesions, BPA may be alternatively considered but not recommended because proximal thrombi cannot be perfectly removed as in PEA. Cases with proximal thrombi were reported to be successfully treated with staged BPA sessions, with rapid increases in pulmonary blood flow indicating perfect revascularization[66,67].
BPA contraindications include iodine allergy because of inevitable use of contrast agents[59], and gadolinium contrast media may be considered as an alternative[68]. Additionally, patients who are unable to undergo RHC should always be excluded. Relative contraindications for BPA procedures were older age, and serious comorbidities such as active infectious diseases[69], severe hepatic or renal dysfunction[69,70], severe chronic obstructive pulmonary disease, bleeding or clotting tendency[71], poorly controlled or uncontrolled diabetes and hypertension[61], careful consideration and caution must be taken when the above conditions exist. In-hospital mortality and overall survival rates after PEA were comparable in patients aged ≥ 70 years and < 70 years[72]. Similarly, hemodynamic improvements, length of intensive care unit and hospital stay, BPA-associated complications, and all-cause mortality were all comparable between younger and elderly patients receiving BPA[58,73,74], suggesting older age is actually not a contraindication for PEA or BPA.
Thromboembolic lesions are classified into five types based on lesion location and angiographic morphology: Ring-like stenosis lesion (band lesion), web lesion, subtotal occlusion lesion, total occlusion lesion (pouch defect), and tortuous lesion (Figure 3)[75,76]. Priority selection of target lesions is as follows: right lung > left lung, inferior lobe > superior or middle lobes, webs or bands > subtotal occlusion > chronic total occlusion > tortuous lesions[76]. Compared to the left, the right lung is given priority owing to easier manipulation, and more blood flow and lesion distribution[53,75,77]. Due to the effect of gravity, the blood flow in the inferior lobe is physiologically larger than in the superior and middle lungs, thus targeting lesions in the lower lobes may enable more prevalent improvements in pulmonary hemodynamics[78]. Chronic subtotal/total occlusion and tortuous lesions have high complication rates (nearly 2% for webs or bands; 15.5% for subtotal occlusion, 6% for total occlusion, and 43.2% for tortuous lesions) and low success rates (nearly 100% for webs or bands, 86.5% for subtotal occlusion, and 52.2% for total occlusion, and 63.6% for tortuous lesions)[59,75], and therefore should be eluded during initial session or when the patient is generally unstable with poor pulmonary hemodynamics (e.g., high mPAP or low cardiac output)[77]. Furthermore, high mPAP before BPA is an independent predictor of the need for mechanical ventilation once lung injury occurs after BPA[79].
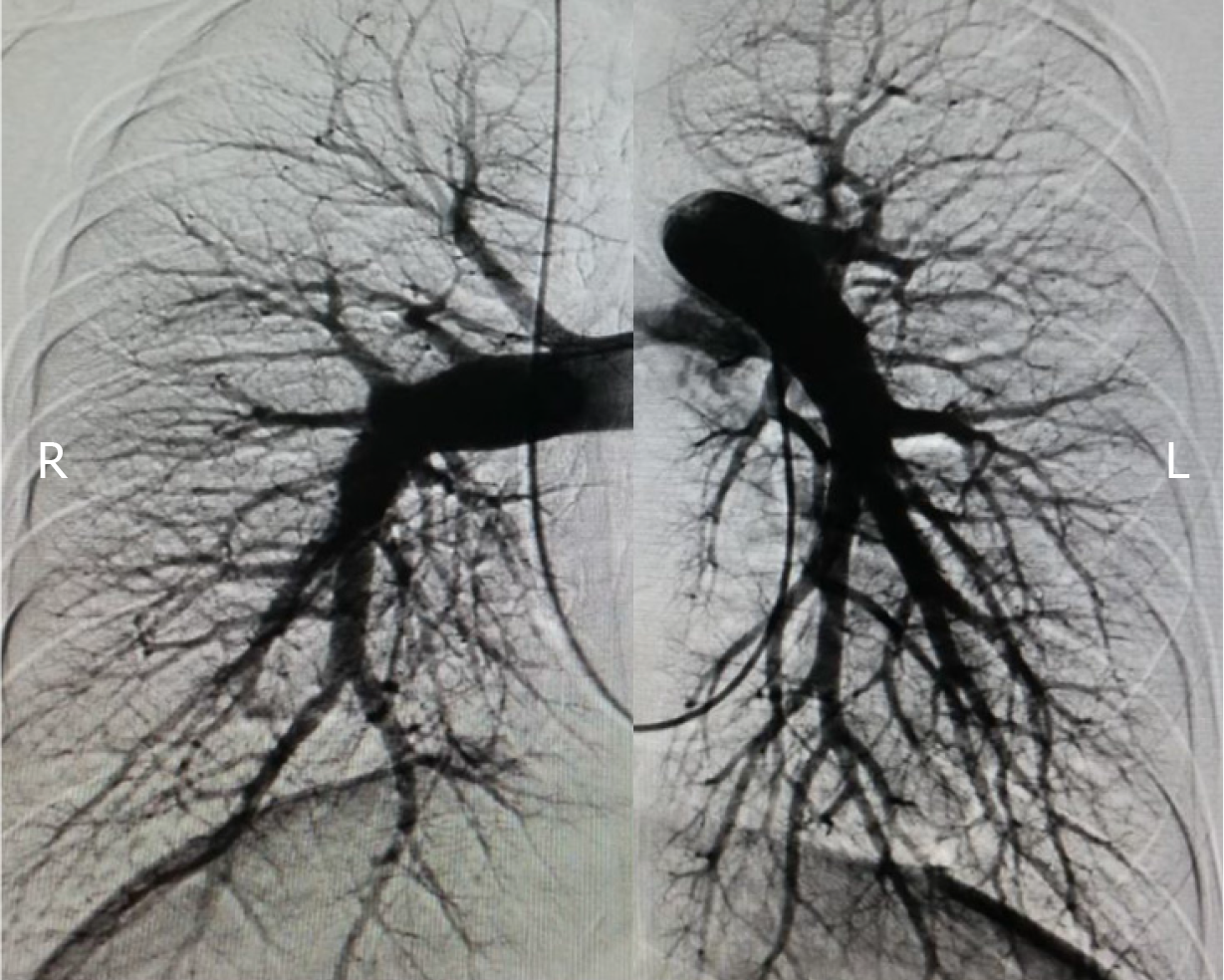
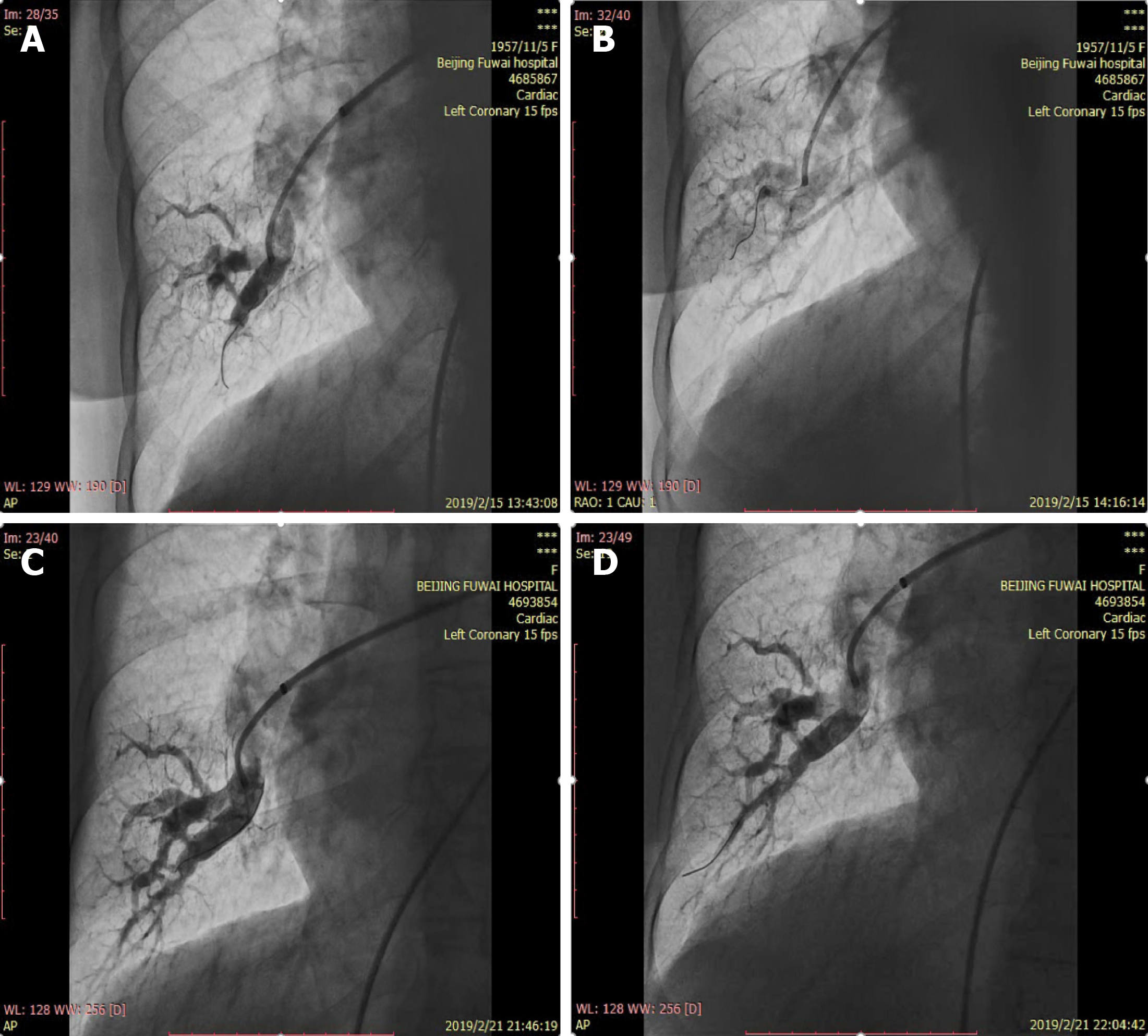
In our center, warfarin is stopped on admission and replaced by low molecular weight heparin to maintain activated clotting time at 250-300 s prior to the procedure. Before RHC and BPA, comprehensive management to stabilize and maintain cardiovascular hemodynamics, right heart function, and nutrition status with necessary pulmonary vasodilators, diuretics and inotropic agents is of great significance for inoperable CTEPH patients. RHC is routinely and preferably approached through the right femoral vein which allows easy manipulation and relatively low radiation exposure. In a few cases, the right jugular vein is an alternative option for those with local infection at the femoral puncture site, inferior vena cava thrombosis or filter implantation. For unilateral pulmonary angiography, 25 mL iodinated contrast media is automatically administered using a power injector through a 6 Fr pigtail angiographic catheter at a rate of 12 mL/s in anterior-posterior and 45-60°C lateral projections (Figure 4), in combination with previous lung perfusion scintigraphy and CTPA to obtain a comprehensive view of the targeted vessel location, lesion types and stenosis degree (Figure 5).
The above pigtail catheter is then replaced by a 260 cm stiff guidewire before insertion of a 70 cm (80 cm for tall or overweight patients) 7 Fr long sheath into the main pulmonary artery tract. A 6 Fr guiding catheter (Multipurpose, Amplatz Left or Judkins Right) is advanced to the lobar pulmonary artery along with the long sheath. Unfractionated heparin (2000 U) is administered after introducing the guiding catheter, with an additional supply (500 U) per hour to maintain an activated clotting time of 200 to 300 s. During the BPA procedure, mask oxygen inhalation is given at a flow rate of 5-10 L/min.
A 0.014-inch wire is crossed to the target lesion, then an appropriate balloon catheter (1.2-6.0 mm) is passed over the selected lesion and inflated with a 1:1 mixture of saline and iodinated contrast agents to 2-14 atmospheres by hand for 5-30 s until fully expanded. To avoid severe complications, a 2.0 mm × 20 mm balloon is positioned over the targeted lesions at initial dilation, and even smaller balloons may be chosen to cross subtotal or total occluded lesions. Inflation pressures are dynamically altered according to balloon and vessel size, and selective angiography with contrast agents is performed with deep breath holds in real time to confirm the effect and to visualize possible vascular perforation or dissection manifested as extravasation or retention of contrast media. Repeated dilations with unchanged or stepwise increased balloon size are performed in case of unresponsive or poorly responsive targeted angiographic vessel (< 50% increase in vessel size). It is worth noting that a single vessel should not be dilated too aggressively to avoid any complications, the effect of dilatation may not be immediately apparent, and the targeted vessel will be spontaneously dilated as blood flows (Figure 6)[80,81]. When to terminate dilatation can be roundly judged from the following points: (1) Increased angiographic diameter of the targeted vessel to near normal size[30]; (2) A grade three angiographic pulmonary flow or visible venous return[61,77,82]; (3) Marked lumen enlargement indicated by OCT or IVUS[83]; or (4) The distal to proximal pressure ratio across the targeted lesion ≥ 0.8[61]. Catheterization hemodynamics ought to be measured again at the end of each BPA session, but pulmonary angiography is not necessarily performed for the sake of possible impairment of renal function caused by contrast agents. A typical BPA session lasts for 1-2 h, and should never exceed 2000 mGy X-ray exposure, 60 min fluoroscopy time, and 300 mL contrast agents. Complications such as RPE, wire perforation and hemoptysis adjudicated by two or more interventional cardiologists should be well recorded. Low molecular weight heparin is restarted 6 h after BPA and replaced on the second day.
After BPA, the patients should be intensively cared for 48 h in case of possible complications or deterioration. Additional BPA with appropriate balloon size depending on vessel diameters is repeated until mPAP ≤ 25 mmHg. The BPA procedure interval varies from one week to several months in different centers[46,58,82,84], we tend to perform 1-2 mo later to avoid possible pulmonary edema due to acute revascularization, and excessive X-ray and contrast media exposure. Although controversies exist, the current therapeutic goal of the BPA procedure is to achieve normalization of hemodynamics (mPAP < 25 mmHg) and oxygen saturation > 95% without using any vasodilators or oxygen supplementation, at least in our center and Okayama Medical Center, the world largest BPA center[85-87]. With staged BPA therapy, it is expected to reduce mPAP and PVR and improve oxygen saturation, thereby improving exercise tolerance, quality of life, and long-term prognosis of CTEPH patients.
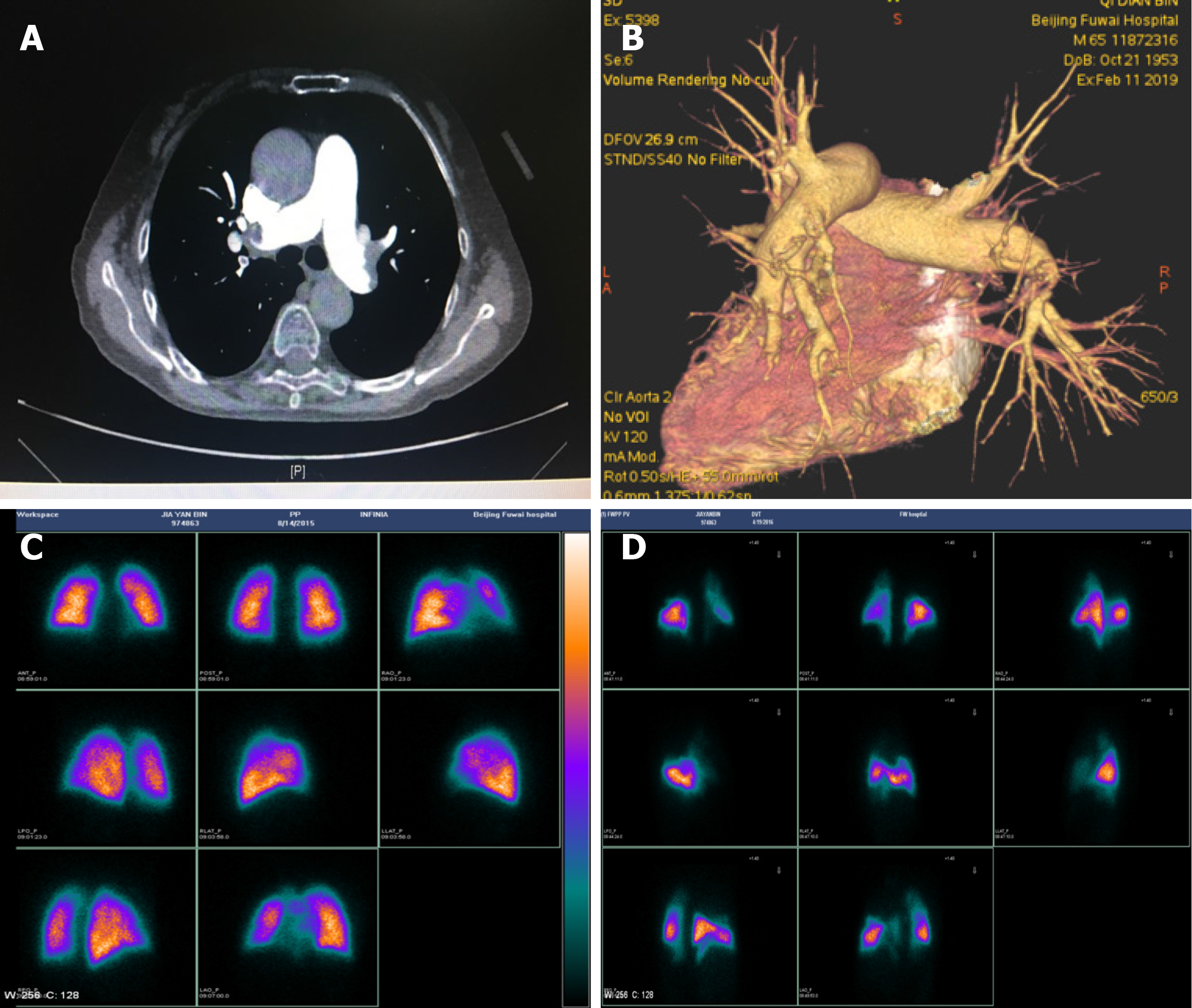
In addition to conventional pulmonary angiography, there are many potential imaging methods that can assist us to accurately assess the lesion location, shape and type, provide real-time dynamic images and videos, and also help guide BPA procedures. However, the cost and time consumed as well as their feasibility, utility, and safety must be considered when using these imaging methods.
OCT is a new imaging modality, which produces optically scattered 2-dimensional (2-D) images showing high-resolution internal tissue microstructure[47]. OCT-guided BPA allows us to precisely assess lesion type and vessel diameter, and facilitate the selection of appropriate balloon size and length, and can also be utilized to evaluate the effectiveness of BPA; however, it may result in potential volume overload and right heart failure[82,88]. Based on morphological features detected by OCT, vessel lesions are categorized into septum, multi-hole with thin wall, multi-hole with thick wall, and mono-hole (Table 1), most of the septum and multi-hole with thin wall types can be easily dilated to achieve a distal to proximal pressure ratio value > 0.8; however, it is quite hard to reach that goal when handling multi-hole with thick wall and mono-hole types, which account for 70% of band lesions. This OCT-based morphological classification helps to differ lesions with good therapeutic responses to BPA and guide a safe and efficient BPA procedure[89]. IVUS is an endovascular imaging technique that uses a miniaturized ultrasound probe to generate sound waves and produce real-time intravascular images, and due to relatively lower resolution compared with OCT, IVUS has limited ability to assess thin organized thrombi, and is mainly applied to determine vessel diameter so as to select the appropriate balloon. In addition, virtual histology IVUS is capable of identifying vascular lesions vulnerable to balloon compression and helps us decide which organized thrombus can be treated to avoid potential pulmonary artery rupture[56]. Angioscopy is a useful modality to visually evaluate various forms of organized thrombus such as white and red thrombus, and may facilitate treatment decision-making and interventional device selection[90-92]. Cone beam computed tomography (CBCT) can provide high-resolution 3-D views of intraluminal organized thrombus in segmental or subsegmental vessels[93]. Representative forms of organized thrombus can be classified into four types according to reconstructed images of CBCT during pulmonary angiography, i.e., type 1: Webs, type 2: Web and slits, type 3: Slits, and type 4: Narrowing or complete occlusion (Table 1). Slit lesions in type 2 and 3 may be easily pushed laterally with dilated balloons, while webs in type 1 and 2 are composed of fibroelastic tissues which are hard to treat with BPA[94,95]. Moreover, CBCT has been reported to be superior to CTPA in precisely detecting subsegmental webs and/or slits[94]. 2-D parametric parenchymal blood flow, 3-D single-photon emission CT/CT fusion imaging and 4-D flow magnetic resonance imaging can help visualize the blood flow of pulmonary arteries, guide the BPA procedure and assess the effects of BPA[96-100].
| Imaging modalities | Types | Description |
| Pulmonary angiography | Ring-like stenosis lesion (band lesion) | Localized lesions with concentric stenosis, as if a ring were put on the vessel |
| Web lesion | Slit, hazy, or abrupt narrowing opacity of the vessel | |
| Subtotal occlusion lesion | Tapered and almost completely occluded, but with subtle and slow flow distal to the obstruction | |
| Total occlusion lesion (pouch defect) | Concaved obstructions with invisible distal vessel | |
| Tortuous lesion | Highly tortuous small vessels distal to subsegmental arteries | |
| Cone beam computed tomography | Webs | Thrombi frequently observed at the bifurcation of branches appear in diverse forms of fenestrated membranes or thick eccentrically situated branching mass |
| Web and slits | Concurrence of proximal web at a bifurcation of branches and distal intravascular fibrous septa (flap-like thin membrane) | |
| Slits | Intravascular fibrous septa alone | |
| Narrowing or complete occlusion | Narrowing or complete occlusion | |
| Optical coherence tomography | Septum | Vessel lumen is separated by a partition into less than 4 components |
| Multi-hole with thin wall | Vessel lumen is separated into more than 5 channels by thin mesh-like flaps | |
| Multi-hole with thick wall | Occupied thrombus with more than 5 channels and thick partition walls | |
| Mono-hole | Occupied thrombus with a single small channel in the lumen | |
| Angioscopy | Mesh thrombus | Organized white thrombus |
| Slit thrombus | Filamentous thrombi | |
| Flap thrombus | Thrombi that almost completely occupy the vessel lumen and block blood flow | |
| Mass-like thrombus | Thrombi that form a solid mass |
The initial exploration of the BPA procedure faced key issues compared with PEA, and BPA was not widely accepted due to relatively few improvements in hemodynamics and a higher incidence of lethal complications and mortality risks[41]. With continuous refinements in BPA techniques by Japanese scholars since 2004[59], compelling evidence has demonstrated the definitely beneficial roles of BPA in safely improving clinical symptoms and pulmonary hemodynamics among inoperable CTEPH patients, which have been comprehensively and excellently summarized in several meta-analyses[101-104]. BPA may also be a new treatment option for selected inoperable symptomatic CTED patients, a group presenting with clinical and diagnostic features of CTEPH but without PH, and small-size observational studies have demonstrated a safe and feasible profile in reducing supplementary oxygen requirement and improving pulmonary hemodynamics, WHO-FC and exercise capacity, but further large studies are needed to confirm these results[64,65].
The first and largest multicenter registry included 308 CTEPH patients who underwent 1408 BPA procedures between November 2004 and March 2013 at 7 institutions in Japan. BPA conferred favorable effects on hemodynamics [mPAP: 43.2 ± 11.0 mmHg vs 24.3 ± 6.4 mmHg; PVR: 853.7 ± 450.7 dyne·s·cm−5vs 359.5 ± 222.6 dyne·s·cm−5; cardiac index: 2.6 ± 0.8 L/(min∙m2) vs 2.9 ± 0.7 L/(min∙m2), all P < 0.001], B-type natriuretic peptide (BNP: 239.5 ± 334.2 pg/mL vs 43.3 ± 76.4 pg/mL, P < 0.001), and exercise tolerance assessed by the 6-min walk distance (6MWD: 318.1 ± 122.1 m vs 401.3 ± 104.8 m, P < 0.001) with maintained efficacy at follow-up and less requirements for PAH-targeted therapy and oxygen supplementation[17]. A more recent study reported the largest monocentric experience of BPA outside Japan, a total of 184 inoperable CTEPH patients underwent 1006 BPA sessions from February 2014 to July 2017, and short-term exercise capacity (6MWD: 396 ± 120 m vs 441 ± 104 m, P < 0.001) and hemodynamics [mPAP: 43.9 ± 9.5 mmHg vs 31.6 ± 9.0 mmHg; PVR: 604 ± 226 dyne·s·cm−5vs 329 ± 177 dyne·s·cm−5; cardiac index: 2.68 ± 0.6 L/(min∙m2) vs 3.07 ± 0.75 L/(min∙m2), all P < 0.001] were all significantly improved by refined BPA, and the safety and efficacy of BPA improved over time, indicating an inevitable learning curve for this complex technique[18]. Taniguchi et al[105] retrospectively evaluated the efficacy and safety of BPA and PEA, and found that 29 inoperable patients who received BPA had mPAP improved from 39.4 ± 6.9 mmHg to 21.3 ± 5.6 mmHg (P < 0.001), PVR from 9.54 to 3.55 Wood units (P < 0.001), and cardiac output from 3.47 ± 0.80 to 4.26 ± 1.15 L/min (P < 0.001), while 24 operable cases who underwent PEA had similar effects with decreased mPAP (44.4 ± 11.0 mmHg vs 21.6 ± 6.7 mmHg, P < 0.001), reduced PVR (9.76 Wood units vs 3.23 Wood units, P < 0.001), and elevated cardiac output (3.35 ± 1.11 L/min vs 4.44 ± 1.58 L/min, P = 0.007). BPA significantly improved hemodynamics and clinical status to a similar extent as PEA.
Non-invasive biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin T (cTnT) are independent predictors of survival in precapillary PH[106,107]. Previous studies observed a significant reduction in plasma NT-proBNP and cTnT several months after the last BPA among patients with inoperable or persistent CTEPH, suggesting improved RV strain after BPA[108-110]. Moreover, NT-proBNP reduction was significantly associated with a decline in mPAP and PVR, and dynamic monitoring might facilitate the identification of BPA non-responders[111]. High-sensitivity cTnT and NT-proBNP significantly and steadily decreased after each BPA session, and baseline cTnT markedly correlated with mPAP, PVR and NT-proBNP, which presumably reflected the alleviation of myocardial injury induced by improved RV afterload after BPA intervention[112].
Cardiopulmonary exercise testing is a reliable pathophysiological tool that can be used to objectively and safely evaluate comprehensive cardiopulmonary function. Impaired exercise capacity and ventilatory efficiency are important poor prognostic factors for CTEPH patients[113]. It was shown that peak oxygen consumption decreased and the minute ventilation/carbon dioxide production slope (VE/VCO2 slope) enhanced as baseline PVR increased. The VE/VCO2 slope diminished significantly early after PEA surgery and was significantly associated with the reduction in PVR[114]. Andreassen et al[107,108] evaluated cardiopulmonary function before and 3 mo after BPA in patients with inoperable or persistent CTEPH and found remarkable improvements in cardiopulmonary exercise testing parameters such as peak oxygen consumption (13.6 ± 5.6 mL/(kg∙min) vs 17.0 ± 6.5 mL/(kg∙min), P < 0.001) and VE/VCO2 slope (41 ± 9 vs 34 ± 8, P = 0.002) after BPA. Importantly, rapid recovery from exercise intolerance and ventilatory inefficiency can be observed as early as one week after BPA[115,116], and CTEPH patients even feel much better, and breathe more deeper and easier during BPA procedures. Supervised home-based pulmonary rehabilitation was reported to substantially improve exercise capacity, leg muscle strength, general physical activity and health-related quality of life with a favorable safety profile, and may be considered to accelerate the recovery of patients with inoperable CTEPH or residual PH after PEA or BPA despite optimal medical therapy[117].
Cytokines such as monocyte chemoattractant protein-1, macrophage inflammatory protein 1α, interleukin-6 (IL-6) and interferon-γ-induced protein-10 were all significantly upregulated in PEA specimens and serum samples of CTEPH patients, moreover, elevated circulating IL-6 and interferon-γ-induced protein-10 correlated well with poor catheter-measured hemodynamics in CTEPH patients[118]. There was a short-term increase in the systemic inflammatory response 24 h after a single BPA session, as evidenced by increased serum levels of IL-6 and high sensitivity C-reactive protein, which significantly decreased 6 mo later[119]. The proinflammatory cytokine high-mobility group box 1 was involved in inflammatory diseases via binding to the receptor for advanced glycation end products (RAGE) and toll-like receptors, plasma levels of soluble and endogenous secretory RAGE were significantly increased in CTEPH patients compared with controls, and soluble RAGE was positively correlated with the tricuspid valvular regurgitation pressure gradient evaluated by echocardiography and decreased immediately after BPA[120,121].
RV dysfunction is closely associated with the poor prognosis of CTEPH[122]. BPA can not only ameliorate hemodynamics but also effectively restore RV remodeling and systolic dysfunction as well as left ventricular (LV) function evaluated by cardiac magnetic resonance in inoperable CTEPH patients, as evidenced by remarkable improvements in RV end-diastolic and end-systolic volume index, RV ejection fraction, RV hypertrophy mass and interventricular septal bowing, LV end-diastolic volume index, and LV stroke volume index with concomitant restoration of hemodynamics and exercise capacity[84,123,124]. Echocardiography assessment such as speckle-tracking echocardiography and 3-D echocardiography indicated RV dyssynchrony, 3-D RV volume, RV ejection fraction, and RV systolic peak strain were all significantly improved, moreover, RV echocardiographic parameters were significantly correlated with clinical characteristics including but not limited to RHC hemodynamics, exercise capacity and BNP level[83,122,125-127].
Every attention must be paid to renal function when performing BPA procedures in CTEPH patients, especially those with renal insufficiency, as the use of contrast agents may cause contrast-induced nephropathy. Interestingly, previous studies investigated the impact of BPA on renal function in CTEPH patients, assessed the changes in creatinine and estimated glomerular filtration rate (eGFR) before and after BPA in renally impaired patients with baseline eGFR < 57 mL/min per 1.73 m2, and found that the improvement in plasma creatinine levels reached a borderline significance and eGFR was significantly increased, indicating that BPA could improve renal function in patients with impaired renal function despite the administration of contrast media[128], and these results were validated by several recent studies[129-131]. BPA could even restore hemodynamics to normal and improve renal function in patients with severe right heart failure complicated with liver and renal failure[70], suggesting that renal insufficiency is not an absolute contraindication for BPA. The mechanisms of such improved renal function may be attributed to remission of venous congestion secondary to reduced right heart failure or revived prerenal failure mediated by increased systemic circulation[128].
An early small-scale study including 12 distal-type CTEPH patients showed 100% survival at 2 years after BPA treatments. Another contemporaneous study at Okayama Medical Center reported a mortality rate of 1.49% in 68 inoperable CTEPH patients during 2.2 ± 1.4 years of follow-up after the final BPA. A retrospective study compared the clinical outcomes of CTEPH patients treated with medical therapy, PEA and BPA, of which the 2-year survival rates were 82.0%, 97.4% and 98.5%, respectively, and the 5-year survival rate after diagnosis was much better in the PEA and BPA interventional groups than in the medical therapy group (98% vs 64%)[57]. However, the small sample size is a common flaw in the above-mentioned studies. Outside Japan, the largest monocentric experience from France involved 1006 BPA sessions, and 7 (3.8) deaths occurred among 184 inoperable CTEPH patients, of which 4 (2.2) died perioperatively within 1 mo after BPA, and the 1- and 3-year survival rate was 97.3% and 95.1%, respectively[18]. In 2017, Aoki et al[132] outlined a single-center experience with 424 BPA sessions from Jul 2009 to Oct 2016, with a 5-year survival rate of 98.4% without any periprocedural death, and only one case died of colon cancer at 13 mo after last BPA. Almost simultaneously, the first and largest multicenter BPA registry results involving 308 patients and 1408 procedures showed overall 1-, 2-, and 3-year survival rates of 96.8%, 96.8%, and 94.5%, respectively[17]. Most recently, an oral presentation by Prof. Hiromi Matsubara during the ESC Congress 2019 reported the latest outcomes of BPA at Okayama Medical Center, the world largest BPA center, from November 2014 to March 2019, and the overall survival rates at 1, 3, 5, and 10 years among 418 CTEPH patients followed up for 6.2 ± 2.3 years were 98.6%, 94.0%, 92.5% and 89.5%, respectively, which seemed superior to PEA with overall survival rates of 86%, 84%, 79%, and 72% at 1, 3, 5, and 10 years, respectively[133].
RPE was once considered a major and fatal complication after BPA in the early stage[41,45,46], indicating BPA should not be performed too aggressively. Therefore, the pulmonary edema predictive scoring index (PEPSI), defined as total change of pulmonary flow grade (PFG) scores in all targeted lesions multiplied by baseline PVR (Wood units), was used to minimize the risk of RPE after BPA, and when using a PEPSI score of 35.4 as the cut-off value, the negative predictive value of RPE was 92.3%[77]. For instance, in cases with baseline PVR of 16 Wood units, the BPA procedure must be discontinued immediately to avoid RPE under the following conditions: (1) The PFG changed from grade 0 to grade 2 after the procedure when treating only one vessel; or (2) Angiographic PFG of each vessel improved by one grade when treating two vessels. Moreover, PEPSI with pressure-wire guided BPA strategy could greatly reduce vessel complications and RPE, especially clinically critical RPE that required ventilation therapy[61]. These strategies have largely helped to reduce RPE occurrence, but seem too stringent and impractical for specialized centers where vessel lesions from 4-10 sites are typically treated by experienced hands within one BPA session[59]. Furthermore, PEPSI with pressure-wire guidance to achieve distal mPAP < 35 mmHg is too complex and requires extra time and money due to the use of pressure wires. Patients with low cardiac output and high PAP at baseline may harbor a higher RPE risk after BPA treatment[45]. Diuretics and oxygen therapy are common approaches to manage RPE, and high-flow nasal cannula therapy up to 50 L/min was reported to relieve RPE after BPA in a patient with a central type lesion[134].
BPA is now considered to rarely cause classical RPE, and the most common complication is lung injury (46%) after BPA, possibly due to wire injury, balloon overdilation, or high-pressure injection of contrast medium[50,75,79]. The previously reported incidence of so-called reperfusion lung injury was as high as 60%, far exceeding the 16%-22% incidence of RPE after PEA surgery[41,46,135,136]. In addition, pulmonary edema is often diffusely distributed, while lung injury is often localized, and high-resolution multidetector CT confirmed that the so-called RPE during BPA was only a focal infiltration at the targeted site[79]. Angiographic extravasation, increased mPAP, and newly developed clinical symptoms such as cough, bloody sputum or hemoptysis, tachycardia, and progressive hypoxia are likely to indicate the occurrence of lung injury manifesting as ground-glass opacity, consolidation, and pleural effusion on high-resolution CT[79]. Minor hemoptysis can resolve spontaneously, once moderate to massive hemoptysis or wire perforation occurs, the bleeding is usually well controlled by neutralizing heparin with protamine or blocking the proximal vessel with a balloon for about 15 min, and after close observation, it can be decided whether to select other vessel lesions for balloon dilatation according to the patient's condition. Preoperative epoprostenol and methylprednisolone, and prophylactic noninvasive positive-pressure ventilation (NPPV) were previously attempted to reduce lung injury occurrence but did not seem to work[46,79]. Once lung injury occurs, severe cases may require NPPV, invasive mechanical ventilation and even extracorporeal membrane oxygenation support[44,46,137,138]. Vascular rupture caused by an overlarge balloon or forceful catheter insertion is a lethal complication during BPA, covered stent placement or coil embolization may be considered when it occurs, but the latter is accompanied by blood flow loss in the embolized vessel[46,139,140].
BPA complications should be clearly defined and uniformly reported, as complication rates are greatly influenced by the definition, thus a new guide to clearly classify complications has been proposed for BPA centers (Table 2)[50]. A systematic review summarized that the early mortality rate of BPA ranged from 0% to 14.3%, the incidence rates of lung injury, hemoptysis and vascular perforation occurred in 7.0%-31.4%, 5.6%-19.6% and 0%-8.0%, respectively[101]. The world largest multicenter registry from Japan described pulmonary injury (17.8%) and hemoptysis (14.0%) as the most frequent complications, pulmonary artery perforation, dissection and rupture accounted for 2.9%, 0.4%, and 0.1%, respectively, and allergic reactions to contrast agents (0.1%) were rarely seen[17]. The recent French BPA experience revealed that lung injury, hemoptysis, pulmonary artery perforation and dissection occurred in 9.1%, 7.1%, 2.8, and 1.9%, respectively, among 1006 BPA sessions, and the incidence of lung injury decreased over time from initial 13.3% to recent 5.9%, clearly indicating that the occurrence of BPA complications depends on center experience[18].
| During the procedure | After the procedure |
| Vascular injury with/without hemoptysis | Lung injury (radiographic opacity with/without hemoptysis, with/without hypoxemia) |
| Wire perforation | Renal dysfunction |
| Balloon over-dilatation | Access site problems |
| High-pressure contrast injection | |
| Vascular dissection | |
| Allergic reaction to contrast | |
| Adverse reaction to conscious sedation/local anesthesia |
Great advances in targeted agents have largely enriched therapeutic options for PAH patients and significantly improved their clinical outcomes[141,142]. CTEPH shares many similarities with PAH in clinical features, hemodynamic status, as well as micro-vasculopathy, thus PAH targeted therapies appear to be attractive for CTEPH patients[15,143]. The beneficial effects of PAH-targeted therapies in inoperable CTEPH have been widely demonstrated in many case series and uncontrolled studies[144,145], but high-level randomized controlled trials (RCTs) are still lacking[12-14,16].
The BENEFIT trial (ClinicalTrials.gov Identifier: NCT00313222) was the first large, double-blind RCT to assess the safety and efficacy of oral dual endothelin receptor antagonist bosentan in either inoperable CTEPH or persistent/recurrent PH patients. Satisfactory tolerability was observed with a significant 24.1% improvement in PVR compared with placebo control, but a non-significant increase of only +2.2 m in 6MWD after a 16-wk course[13]. Irrespective of background PAH-targeted therapy, the dual endothelin-receptor antagonist macitentan significantly improved PVR (-16%), 6MWD (+34 m), cardiac output and NT-proBNP at week 16 among inoperable CTEPH patients[14]. The CHEST-1 trial (ClinicalTrials.gov Identifier: NCT00855465), a phase 3, multicenter, double-blind RCT in 261 inoperable CTEPH or persistent/recurrent PH, demonstrated a large benefit of the soluble guanylate cyclase stimulator riociguat (titrated up to 2.5 mg tid) in improving 6MWD (+46 m) and PVR (-31%) at the 16-wk follow-up[12]. The CTREPH trial (ClinicalTrials.gov Identifier: NCT01416636) was a multicenter, double-blind RCT aiming to evaluate the efficacy and tolerability of subcutaneously administered treprostinil for severe non-operable or persistent /recurrent CTEPH over a treatment period of 24 wk, 105 patients were 1:1 randomly allocated to low-dose [3 ng/(kg∙min) at week 12] and high-dose [30 ng/(kg∙min) at week 12] groups, and subcutaneous treprostinil treatment improved exercise capacity (6MWD: +44.98 m in the high-dose group vs +4.29 m in the low-dose group), but serious adverse events were observed in both the high-dose (17%) and low-dose (19%) groups[16,146]. To date, riociguat remains the only United States Food and Drug Administration approved drug indicated for inoperable, persistent or recurrent CTEPH.
Notably, the use of targeted therapy as a bridge to PEA is controversial and may delay timely surgical referral, as medical therapy cannot remove intraluminal thrombus in pulmonary arteries as PEA does and its therapeutic effect is limited[50]. Preoperative targeted therapy to maintain high-risk CTEPH patients in a stable state could reduce the risk of BPA and the occurrence of postoperative complications[147,148]. BPA has been reported to be safe and feasible as a bridging or one-stage procedure to PEA in high surgical risk patients who have both proximal and distal lesions[149-151]. A hybrid approach of PEA with additional BPA is reasonably practical for patients with residual or recurrent PH after PEA, and sequential hybrid therapy safely and effectively lowered mPAP (18.7 ± 1.7 mmHg vs 30.2 ± 3.2 mmHg, P = 0.008), improved residual symptoms and exercise capacity (429 ± 38 m vs 319 ± 22 m, P = 0.028) compared with PEA surgery alone, and further large and prospective studies are needed to confirm the long-term safety and efficacy of additional BPA and determine the optimal timing of additional BPA after PEA[62,152,153].
The MR BPA study (UMIN Clinical Trials Registry ID: UMIN000019549) is a Japanese multicenter, prospective, open-label RCT comparing the efficacy and safety of BPA and riociguat for inoperable CTEPH patients enrolled from January 2016 to October 2019 whose primary endpoint is the change from baseline in mPAP over a 12-mo treatment course, several clinical parameters such as 6MWD, PVR, pulmonary function, and quality of life assessment are included as secondary endpoints, furthermore, the costs of health insurance resource between the two groups will also be discussed, the results of the study are expected to be available in the coming months[154]. A similar RCT named the RACE study (ClinicalTrials.gov Identifier: NCT02634203) was initiated synchronously in France in January 2016, and 105 inoperable CTEPH patients with baseline PVR > 4 Wood units were randomized (52 to BPA and 53 to riociguat) to 26 wk of intervention in 14 French centers between January 2016 and January 2019, with the primary endpoint being relative change from baseline in PVR[155]. A preliminary report from ERS 2019 showed that BPA was superior to riociguat in reducing PVR (59% vs 32%, P < 0.0001) and BNP (BPA vs riociguat: 67% reduction), however, the change in 6MWD was not significant (BPA 50 m vs riociguat 44 m, P = 0.62), and the proportion of patients with treatment-related adverse events was higher in the BPA group than in the riociguat group (42% vs 9%), the formal results are soon to be published[156].
The International BPA Registry (ClinicalTrials.gov Identifier: NCT03245268) is a prospective, multicenter and observational study led by Professor Nick Kim to assess the efficacy and safety of BPA intervention in CTEPH patients deemed ineligible for PEA, which will last approximately 4 years from March 2018 with each patient followed for a minimum of 2 years, and is expected to end by March 2022. The Japan Multicenter Registry for CTEPH (CTEPH AC Registry, https://cteph-registry.jp/en/), beginning in November 2018, is in progress to create solid real-world evidence for pulmonary vasodilators, PEA and BPA, with over 715 patients from 36 institutions registered as of April 20, 2020. The hemodynamic effects of BPA at rest and during exercise (EXPERT-BPA, ClinicalTrials.gov Identifier: NCT04052243) and its impact on sleep disordered breathing (ClinicalTrials.gov Identifier: NCT03074539) are also being investigated. Our center, with more than 2000 referrals of patients with pulmonary vascular diseases and over 100 BPA sessions performed per year, launched the first prospective, multicenter, long-term observational study in May 2018, and aimed to evaluate the safety and efficacy of BPA in patients with inoperable CTEPH and persistent/recurrent PH after PEA in China (ClinicalTrials.gov Identifier: NCT04206852).
The true incidence of CTEPH remains unclear, with approximately 1%-5% of patients progressing to CTEPH after acute pulmonary embolism[157,158]. The pathophysiology of CTEPH is not yet fully understood, and the lack of appropriate experimental animal models is a major challenge, and in-depth exploration of the evolution of fresh thrombi into organized thrombi and the development of pulmonary microangiopathy may help to fundamentally prevent the occurrence and deterioration of CTEPH.
There are limited multicenter, prospective, randomized controlled studies on the application of PAH-targeted agents in CTEPH. Numerous observational studies have shown that targeted therapy is effective for CTEPH, but the effect of current approved drug riociguat is extremely limited and non-curative with an extremely high price, at least in China. Despite the lack of sufficient evidence, targeted therapies have been routinely prescribed and commonly seen among CTEPH patients treated with PEA or BPA. Intensive targeted therapy before and after BPA or PEA may contribute to safety and efficacy implementation, as these treatments are not mutually exclusive and can be used in combination or sequentially, but their timing and cost-effectiveness are important factors to be considered and should be further analyzed. A multicenter, prospective RCT (ClinicalTrials.gov Identifier: NCT03273257) assessing the efficacy of riociguat as PEA bridging therapy compared to placebo in operable CTEPH patients with high PVR is ongoing. The latest results from the RACE study suggested an encouraging superiority of BPA over riociguat, yet the effects of prostacyclin receptor agonists such as selexipag (ClinicalTrials.gov Identifier: NCT03689244) and ralinepag in CTEPH patients remain unknown.
PEA remains the first choice for patients with accessible lesions, and all patients should be managed in specialized centers due to the extremely high demands on surgical skills and teams. For high-risk patients, preoperative intensive targeted therapy or BPA bridging may stabilize the patient's overall condition at an optimal level, which helps to reduce the risk of PEA surgery. Additional BPA as hybrid therapy is a new option for patients with residual/recurrent PH after surgery. Indeed, the order and optimal timing of BPA in relation to PEA are not clearly conclusive to date.
There is an encouraging body of evidence demonstrating the efficacy and safety of BPA, which can significantly improve hemodynamics, oxygen saturation, and exercise capacity. BPA shows absolute advantages over PEA for distal lesions and residual/recurrent PH, however, its efficacy in patients with proximal lesions and CTED still needs further investigation. Therapeutic strategies, efficacy, and safety of BPA vary greatly among institutions, and interventionist experience, technical skills, and interventional instruments all greatly affect the short- and long-term effects of BPA as well as the occurrence of complications, and there is an urgent need for standardized BPA procedures and a unified definition of complications. Gentle manipulation of the guiding wire and catheter and appropriate pressure of balloon dilation are particularly vital to reduce the occurrence of complications. The safety and efficacy of measures such as stent implantation, coils and NPPV need to be addressed. Imaging techniques such as OCT and IVUS facilitate the selection of vessel lesions and the evaluation of BPA effects and help to reduce complications, but time and cost must be considered. The exact restenosis rate as well as disease progression after BPA is unknown, although current evidence suggests that restenosis rarely occurs[46]. BPA is performed in a staged manner, but the optimal BPA interval is unclear. After BPA, it is undecided whether CTEPH patients need to continue targeted therapy while achieving normal hemodynamics. Although PEA and BPA have different requirements for lesion accessibility, there is no head-to-head comparison, and a recent single-center experience from Okayama Medical Center suggests that BPA has comparable long-term prognosis to PEA.
A multidisciplinary team involving radiologists, anesthesiologists, PEA surgeons, BPA interventionists, and PH experts can provide precise individualized treatment strategies for CTEPH patients[159,160]. PEA remains the preferred therapeutic approach that should be offered to all eligible operable patients. After over 30 years of rapid development and refinements, BPA has shown excellent efficacy with greatly reduced incidence of complications, and has been widely accepted as a promising treatment strategy for patients with inoperable CTEPH and residual/recurrent PH, but further large-scale, prospective, multicenter, randomized controlled studies are needed to further clarify the optimal selection of patients and lesions, therapeutic strategies, management of complications and long-term prognosis.
We thank Professor Hiromi Matsubara and Hiroto Shimokawahara, Department of Clinical Science, National Hospital Organization Okayama Medical Center, Okayama, Japan, for their directions in BPA procedures.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosokawa K S-Editor: Ma RY L-Editor: Webster JR E-Editor: Xing YX
| 1. | Madani M, Ogo T, Simonneau G. The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev. 2017;26:170105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Lewczuk J, Piszko P, Jagas J, Porada A, Wójciak S, Sobkowicz B, Wrabec K. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119:818-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 516] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:1605-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97-103; discussion 103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 7. | Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141:383-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Hoeper MM, Madani MM, Nakanishi N, Meyer B, Cebotari S, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med. 2014;2:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Jenkins D, Madani M, Fadel E, D'Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 10. | Ruigrok D, Braams NJ, Nossent EJ, Bonta PI, Boonstra A, Lely RJ, Klok E, Vonk-Noordegraaf A, Symersky P, Jan Bogaard H, Meijboom LJ. EXPRESS: Dynamic vascular changes in CTEPH after pulmonary endarterectomy. Pulm Circ. 2020;2045894020907883. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Montani D, Chaumais MC, Guignabert C, Günther S, Girerd B, Jaïs X, Algalarrondo V, Price LC, Savale L, Sitbon O, Simonneau G, Humbert M. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141:172-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C; CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 932] [Cited by in F6Publishing: 892] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 13. | Jaïs X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ; Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension Study Group. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 14. | Ghofrani HA, Simonneau G, D'Armini AM, Fedullo P, Howard LS, Jaïs X, Jenkins DP, Jing ZC, Madani MM, Martin N, Mayer E, Papadakis K, Richard D, Kim NH; MERIT study investigators. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5:785-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Yu X, Jin Q, Luo Q, Zhao Z, Zhao Q, Yan L, Liu Z. Advances in targeted therapy for chronic thromboembolic pulmonary hypertension. Heart Fail Rev. 2019;24:949-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Sadushi-Kolici R, Jansa P, Kopec G, Torbicki A, Skoro-Sajer N, Campean IA, Halank M, Simkova I, Karlocai K, Steringer-Mascherbauer R, Samarzija M, Salobir B, Klepetko W, Lindner J, Lang IM. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med. 2019;7:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2017;10:e004029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, Mercier O, Fabre D, Parent F, Jevnikar M, Montani D, Savale L, Sitbon O, Fadel E, Humbert M, Simonneau G. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1802095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 19. | Lang IM, Dorfmüller P, Vonk Noordegraaf A. The Pathobiology of Chronic Thromboembolic Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13 Suppl 3:S215-S221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 21. | Ackermann M, Gaumann A, Mentzer SJ, Hinrichs JB, Warnecke G, Hoeper MM, Braubach P, Kuehnel M, Maegel L, Jonigk D. Plexiform Vasculopathy in Chronic Thromboembolic Pulmonary Hypertension. Am J Respir Crit Care Med. 2017;196:e48-e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sakao S, Hao H, Tanabe N, Kasahara Y, Kurosu K, Tatsumi K. Endothelial-like cells in chronic thromboembolic pulmonary hypertension: crosstalk with myofibroblast-like cells. Respir Res. 2011;12:109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Wang L, Gan HL, Liu Y, Gu S, Li J, Guo LJ, Liu J, Wang Y, Wang YX, Zhang ZF, Wang J, Wang C. The distinguishing cellular features of pulmonary artery smooth muscle cells from chronic thromboembolic pulmonary hypertension patients. Exp Lung Res. 2013;39:349-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Xi Q, Liu Z, Song Y, Gan H, Huang Z, Luo Q, Zhao Z. Proteomic Analyses of Endarterectomized Tissues from Patients with Chronic Thromboembolic Pulmonary Hypertension. Cardiology. 2020;145:48-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yan L, Li X, Liu Z, Zhao Z, Luo Q, Zhao Q, Jin Q, Yu X, Zhang Y. Research progress on the pathogenesis of CTEPH. Heart Fail Rev. 2019;24:1031-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Jin Q, Zhao Z, Zhao Q, Yu X, Yan L, Zhang Y, Luo Q, Liu Z. Long noncoding RNAs: emerging roles in pulmonary hypertension. Heart Fail Rev. 2019; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Kapitan KS, Clausen JL, Moser KM. Gas exchange in chronic thromboembolism after pulmonary thromboendarterectomy. Chest. 1990;98:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Martin EC, Diamond NG, Casarella WJ. Percutaneous Transluminal Angioplasty in Non-Atheroscklerotic Disease. Radiology. 1980;135:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Lock JE, Niemi T, Einzig S, Amplatz K, Burke B, Bass JL. Transvenous angioplasty of experimental branch pulmonary artery stenosis in newborn lambs. Circulation. 1981;64:886-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Lock JE, Castaneda-Zuniga WR, Fuhrman BP, Bass JL. Balloon dilation angioplasty of hypoplastic and stenotic pulmonary arteries. Circulation. 1983;67:962-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 196] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Rocchini AP, Kveselis D, Dick M, Crowley D, Snider AR, Rosenthal A. Use of balloon angioplasty to treat peripheral pulmonary stenosis. Am J Cardiol. 1984;54:1069-1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Rocchini AP, Kveselis D. The use of balloon angioplasty in the pediatric patient. Pediatr Clin North Am. 1984;31:1293-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Edwards BS, Lucas RV, Lock JE, Edwards JE. Morphologic changes in the pulmonary arteries after percutaneous balloon angioplasty for pulmonary arterial stenosis. Circulation. 1985;71:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Di Sessa TG, Yeatman LA, Williams RG, Lois JF, Friedman WF, Laks H. Thrombosis complicating balloon angioplasty of left pulmonary artery stenosis after Fontan's procedure: successful treatment with intravenous streptokinase. Am J Cardiol. 1985;55:610-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Bass JL. Percutaneous balloon dilation angioplasty of pulmonary artery branch stenosis. Cardiovasc Intervent Radiol. 1986;9:299-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Hoekenga DE, Stevens GF, Ball WS. Percutaneous angioplasty for peripheral pulmonary stenosis in an adult. Am J Cardiol. 1987;59:188-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Snyder MS, Sos T, Levin AR, Engle MA. Transluminal angioplasty of a stenotic Potts shunt and pulmonary arterial branch stenosis. Am Heart J. 1987;113:198-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | D'Orsogna L, Sandor GG, Culham JA, Patterson M. Successful balloon angioplasty of peripheral pulmonary stenosis in Williams syndrome. Am Heart J. 1987;114:647-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Handa K, Sasaki Y, Kiyonaga A, Fujino M, Hiroki T, Arakawa K. Acute pulmonary thromboembolism treated successfully by balloon angioplasty--a case report. Angiology. 1988;39:775-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Voorburg JA, Cats VM, Buis B, Bruschke AV. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest. 1988;94:1249-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 327] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Pitton MB, Herber S, Mayer E, Thelen M. Pulmonary balloon angioplasty of chronic thromboembolic pulmonary hypertension (CTEPH) in surgically inaccessible cases. Rofo. 2003;175:631-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | de Gregorio MA, Laborda A, Ortas R, Higuera T, Gómez-Arrue J, Medrano J, Mainar A. [Image-guided minimally invasive treatment of pulmonary arterial hypertension due to embolic disease]. Arch Bronconeumol. 2008;44:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Moriyama K, Sugiyama S, Uzawa K, Kotani M, Satoh T, Yorozu T. Noninvasive Positive Pressure Ventilation against Reperfusion Pulmonary Edema following Percutaneous Transluminal Pulmonary Angioplasty. Case Rep Anesthesiol. 2011;2011:204538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, Tamura Y, Ando M, Fukuda K, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:756-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 46. | Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 47. | Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, Tatebe S, Miyamichi-Yamamoto S, Shimokawa H. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76:485-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 48. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1826] [Cited by in F6Publishing: 1929] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 49. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3636] [Cited by in F6Publishing: 3916] [Article Influence: 435.1] [Reference Citation Analysis (0)] |
| 50. | Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1801915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 51. | Kitani M, Ogawa A, Sarashina T, Yamadori I, Matsubara H. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2014;7:857-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Ogawa A, Kitani M, Mizoguchi H, Munemasa M, Matsuo K, Yamadori I, Andou A, Matsubara H. Pulmonary microvascular remodeling after balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Intern Med. 2014;53:729-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Shimokawahara H, Ogawa A, Mizoguchi H, Yagi H, Ikemiyagi H, Matsubara H. Vessel Stretching Is a Cause of Lumen Enlargement Immediately After Balloon Pulmonary Angioplasty: Intravascular Ultrasound Analysis in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ Cardiovasc Interv. 2018;11:e006010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Räber L, Ueki Y, Lang IM. Balloon pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. EuroIntervention. 2019;15:e814-e815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Araszkiewicz A, Jankiewicz S, Janus M, Łanocha M, Mularek-Kubzdela T, Lesiak M. Optical coherence tomography reveals the mechanisms of balloon pulmonary angioplasty in inoperable chronic thromboembolic pulmonary hypertension. Cardiol J. 2017;24:334-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Kopeć G, Waligóra M, Stępniewski J, Żmudka K, Podolec P, Matsubara H. In vivo characterization of changes in composition of organized thrombus in patient with chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Int J Cardiol. 2015;186:279-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Inami T, Kataoka M, Ando M, Fukuda K, Yoshino H, Satoh T. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS One. 2014;9:e94587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Yanagisawa R, Kataoka M, Inami T, Shimura N, Ishiguro H, Fukuda K, Yoshino H, Satoh T. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol. 2014;175:285-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Ogawa A, Matsubara H. Balloon Pulmonary Angioplasty: A Treatment Option for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Front Cardiovasc Med. 2015;2:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, Ito H, Kuwana M, Matsubara H, Momomura SI, Nishimura M, Ogino H, Satoh T, Shimokawa H, Yamauchi-Takihara K, Tatsumi K, Ishibashi-Ueda H, Yamada N, Yoshida S, Abe K, Ogawa A, Ogo T, Kasai T, Kataoka M, Kawakami T, Kogaki S, Nakamura M, Nakayama T, Nishizaki M, Sugimura K, Tanabe N, Tsujino I, Yao A, Akasaka T, Ando M, Kimura T, Kuriyama T, Nakanishi N, Nakanishi T, Tsutsui H; Japanese Circulation Society and the Japanese Pulmonary Circulation and Pulmonary Hypertension Society Joint Working Group. Guidelines for the Treatment of Pulmonary Hypertension (JCS 2017/JPCPHS 2017). Circ J. 2019;83:842-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 61. | Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Fukuda K, Yoshino H, Satoh T. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2014;7:1297-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Higuchi Y, Ando M, Fukuda K, Yoshino H, Satoh T. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015;183:138-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 63. | Araszkiewicz A, Darocha S, Pietrasik A, Pietura R, Jankiewicz S, Banaszkiewicz M, Sławek-Szmyt S, Biederman A, Mularek-Kubzdela T, Lesiak M, Torbicki A, Kurzyna M. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2019;278:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 64. | Wiedenroth CB, Olsson KM, Guth S, Breithecker A, Haas M, Kamp JC, Fuge J, Hinrichs JB, Roller F, Hamm CW, Mayer E, Ghofrani HA, Meyer BC, Liebetrau C. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ. 2018;8:2045893217753122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Inami T, Kataoka M, Kikuchi H, Goda A, Satoh T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol. 2019;289:116-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Minatsuki S, Kiyosue A, Kodera S, Hara T, Saito A, Maki H, Hatano M, Takimoto E, Ando M, Komuro I. Effectiveness of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension despite having lesion types suitable for surgical treatment. J Cardiol. 2020;75:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Ishiguro H, Kataoka M, Inami T, Yanagisawa R, Shimura N, Taguchi H, Kohshoh H, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for central-type chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2013;6:1212-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Nakamura T, Ogo T, Tsuji A, Fukui S, Fukuda T, Tahara N, Fukumoto Y, Yasuda S, Ogawa H, Nakanishi N. Successful balloon pulmonary angioplasty with gadolinium contrast media for a patient with chronic thromboembolic pulmonary hypertension and iodine allergy. Respir Med Case Rep. 2016;17:75-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Tsuji A, Ogo T, Demachi J, Ono Y, Sanda Y, Morita Y, Fukuda T, Nakanishi N. Rescue balloon pulmonary angioplasty in a rapidly deteriorating chronic thromboembolic pulmonary hypertension patient with liver failure and refractory infection. Pulm Circ. 2014;4:142-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Inami T, Kataoka M, Ishiguro H, Yanagisawa R, Shimura N, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension with severe right heart failure. Am J Respir Crit Care Med. 2014;189:1437-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Sueoka J, Kataoka M, Shimura N, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Fukuda K, Yoshino H, Satoh T. Therapeutic efficacy after percutaneous transluminal pulmonary angioplasty in CTEPH with and without clotting disorder according to anti-cardiolipin antibody. Int J Cardiol. 2015;201:271-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Berman M, Hardman G, Sharples L, Pepke-Zaba J, Sheares K, Tsui S, Dunning J, Jenkins DP. Pulmonary endarterectomy: outcomes in patients aged >70. Eur J Cardiothorac Surg. 2012;41:e154-e160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Yamagata Y, Ikeda S, Nakata T, Yonekura T, Koga S, Muroya T, Koide Y, Kawano H, Maemura K. Balloon pulmonary angioplasty is effective for treating peripheral-type chronic thromboembolic pulmonary hypertension in elderly patients. Geriatr Gerontol Int. 2018;18:678-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Roik M, Wretowski D, Łabyk A, Irzyk K, Lichodziejewska B, Dzikowska-Diduch O, Piotrowska-Kownacka D, Pruszczyk P. Refined balloon pulmonary angioplasty-A therapeutic option in very elderly patients with chronic thromboembolic pulmonary hypertension. J Interv Cardiol. 2017;30:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Kawakami T, Ogawa A, Miyaji K, Mizoguchi H, Shimokawahara H, Naito T, Oka T, Yunoki K, Munemasa M, Matsubara H. Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty. Circ Cardiovasc Interv. 2016;9:e003318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 76. | Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T. Balloon Pulmonary Angioplasty (Percutaneous Transluminal Pulmonary Angioplasty) for Chronic Thromboembolic Pulmonary Hypertension: A Japanese Perspective. JACC Cardiovasc Interv. 2019;12:1382-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Taguchi H, Fukuda K, Yoshino H, Satoh T. Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv. 2013;6:725-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 78. | Akizuki M, Serizawa N, Ueno A, Adachi T, Hagiwara N. Effect of Balloon Pulmonary Angioplasty on Respiratory Function in Patients With Chronic Thromboembolic Pulmonary Hypertension. Chest. 2017;151:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Ejiri K, Ogawa A, Fujii S, Ito H, Matsubara H. Vascular Injury Is a Major Cause of Lung Injury After Balloon Pulmonary Angioplasty in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ Cardiovasc Interv. 2018;11:e005884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Hosokawa K, Abe K, Oi K, Mukai Y, Hirooka Y, Sunagawa K. Negative acute hemodynamic response to balloon pulmonary angioplasty does not predicate the long-term outcome in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015;188:81-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Nagayoshi S, Ogawa A, Matsubara H. Spontaneous enlargement of pulmonary artery after successful balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2016;12:e1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Bouvaist H, Thony F, Jondot M, Camara B, Jais X, Pison C. Balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2014;23:393-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Tsugu T, Murata M, Kawakami T, Yasuda R, Tokuda H, Minakata Y, Tamura Y, Kataoka M, Hayashida K, Tsuruta H, Maekawa Y, Inoue S, Fukuda K. Significance of echocardiographic assessment for right ventricular function after balloon pulmonary angioplasty in patients with chronic thromboembolic induced pulmonary hypertension. Am J Cardiol. 2015;115:256-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | Fukui S, Ogo T, Morita Y, Tsuji A, Tateishi E, Ozaki K, Sanda Y, Fukuda T, Yasuda S, Ogawa H, Nakanishi N. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J. 2014;43:1394-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 85. | Matsubara H, Ogawa A. A long way to go after the initial experience with balloon pulmonary angioplasty. Eur Respir J. 2017;49:1700718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Kawakami T, Ogawa A, Miyaji K, Mizoguchi H, Shimokawahara H, Naito T, Oka T, Yunoki K, Munemasa M, Matsubara H. Response by Kawakami et al to Letter Regarding Article, "Novel Angiographic Classification of Each Vascular Lesion in Chronic Thromboembolic Pulmonary Hypertension Based on Selective Angiogram and Results of Balloon Pulmonary Angioplasty". Circ Cardiovasc Interv. 2017;10:e004962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Ogawa A, Matsubara H. After the Dawn - Balloon Pulmonary Angioplasty for Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ J. 2018;82:1222-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Roik M, Wretowski D, Łabyk A, Kostrubiec M, Rowiński O, Pruszczyk P. Optical coherence tomography of inoperable chronic thromboembolic pulmonary hypertension treated with refined balloon pulmonary angioplasty. Pol Arch Med Wewn. 2014;124:742-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Inohara T, Kawakami T, Kataoka M, Yamamoto M, Kimura M, Kanazawa H, Yuasa S, Hayashida K, Maekawa Y, Fukuda K. Lesion morphological classification by OCT to predict therapeutic efficacy after balloon pulmonary angioplasty in CTEPH. Int J Cardiol. 2015;197:23-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Nakanishi N, Nakamura T, Yamano T, Shiraishi H, Matoba S, Matsumuro A, Shirayama T. Angioscopic observation in chronic thromboembolic pulmonary hypertension before and after balloon pulmonary angioplasty. J Cardiovasc Med (Hagerstown). 2016;17 Suppl 2:e129-e131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Takano T, Ozaki K, Hoyano M, Yanagawa T, Kashimura T, Minamino T. Angioscopic findings during balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther. 2019; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Nakanishi N, Fukai K, Tsubata H, Ogata T, Zen K, Nakamura T, Yamano T, Shiraishi H, Shirayama T, Matoba S. Angioscopic Evaluation During Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2019;28:655-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Ogo T, Fukuda T, Tsuji A, Fukui S, Ueda J, Sanda Y, Morita Y, Asano R, Konagai N, Yasuda S. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur J Radiol. 2017;89:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Sugiyama M, Fukuda T, Sanda Y, Morita Y, Higashi M, Ogo T, Tsuji A, Demachi J, Nakanishi N, Naito H. Organized thrombus in pulmonary arteries in patients with chronic thromboembolic pulmonary hypertension; imaging with cone beam computed tomography. Jpn J Radiol. 2014;32:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Fukuda T, Ogo T, Nakanishi N, Ueda J, Sanda Y, Morita Y, Sugiyama M, Fukui S, Tsuji A, Naito H. Evaluation of organized thrombus in distal pulmonary arteries in patients with chronic thromboembolic pulmonary hypertension using cone-beam computed tomography. Jpn J Radiol. 2016;34:423-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Maschke SK, Winther HMB, Meine T, Werncke T, Olsson KM, Hoeper MM, Baumgart J, Wacker FK, Meyer BC, Renne J, Hinrichs JB. Evaluation of a newly developed 2D parametric parenchymal blood flow technique with an automated vessel suppression algorithm in patients with chronic thromboembolic pulmonary hypertension undergoing balloon pulmonary angioplasty. Clin Radiol. 2019;74:437-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Hosokawa K, Abe K, Kashihara S, Tsutsui H. 3-Dimensional SPECT/CT Fusion Imaging-Guided Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. JACC Cardiovasc Interv. 2017;10:e193-e194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 98. | Ota H, Sugimura K, Miura M, Shimokawa H. Four-dimensional flow magnetic resonance imaging visualizes drastic change in vortex flow in the main pulmonary artery after percutaneous transluminal pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Heart J. 2015;36:1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Kawakami T, Kataoka M, Nakahara T, Yamada Y, Takei M, Jinzaki M, Fukuda K. Usefulness of 3D SPECT/CT fusion image in CTEPH. Int J Cardiol. 2015;194:39-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Maschke SK, Renne J, Werncke T, Olsson KM, Hoeper MM, Wacker FK, Meyer BC, Hinrichs JB. Chronic thromboembolic pulmonary hypertension: Evaluation of 2D-perfusion angiography in patients who undergo balloon pulmonary angioplasty. Eur Radiol. 2017;27:4264-4270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Tanabe N, Kawakami T, Satoh T, Matsubara H, Nakanishi N, Ogino H, Tamura Y, Tsujino I, Ogawa A, Sakao S, Nishizaki M, Ishida K, Ichimura Y, Yoshida M, Tatsumi K. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A systematic review. Respir Investig. 2018;56:332-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Khan MS, Amin E, Memon MM, Yamani N, Siddiqi TJ, Khan SU, Murad MH, Mookadam F, Figueredo VM, Doukky R, Benza RL, Krasuski RA. Meta-analysis of use of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2019;291:134-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Wang W, Wen L, Song Z, Shi W, Wang K, Huang W. Balloon pulmonary angioplasty vs riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Clin Cardiol. 2019;42:741-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Zoppellaro G, Badawy MR, Squizzato A, Denas G, Tarantini G, Pengo V. Balloon Pulmonary Angioplasty in Patients With Chronic Thromboembolic Pulmonary Hypertension - A Systematic Review and Meta-Analysis. Circ J. 2019;83:1660-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 105. | Taniguchi Y, Miyagawa K, Nakayama K, Kinutani H, Shinke T, Okada K, Okita Y, Hirata KI, Emoto N. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10:518-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 106. | Torbicki A, Kurzyna M, Kuca P, Fijałkowska A, Sikora J, Florczyk M, Pruszczyk P, Burakowski J, Wawrzyńska L. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation. 2003;108:844-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 107. | Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98:525-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 108. | Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99:1415-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 109. | Kimura M, Kohno T, Kawakami T, Kataoka M, Tsugu T, Akita K, Isobe S, Itabashi Y, Maekawa Y, Murata M, Fukuda K. Midterm Effect of Balloon Pulmonary Angioplasty on Hemodynamics and Subclinical Myocardial Damage in Chronic Thromboembolic Pulmonary Hypertension. Can J Cardiol. 2017;33:463-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Kimura M, Kohno T, Kawakami T, Kataoka M, Inohara T, Takei M, Tsugu T, Murata M, Maekawa Y, Fukuda K. Balloon pulmonary angioplasty attenuates ongoing myocardial damage in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2016;207:387-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Kriechbaum SD, Wiedenroth CB, Wolter JS, Hütz R, Haas M, Breithecker A, Roller FC, Keller T, Guth S, Rolf A, Hamm CW, Mayer E, Liebetrau C. N-terminal pro-B-type natriuretic peptide for monitoring after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2018;37:639-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Kriechbaum SD, Wiedenroth CB, Keller T, Wolter JS, Ajnwojner R, Peters K, Haas MA, Roller FC, Breithecker A, Rieth AJ, Guth S, Rolf A, Bandorski D, Hamm CW, Mayer E, Liebetrau C. Dynamics of high-sensitivity cardiac troponin T during therapy with balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. PLoS One. 2018;13:e0204683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Richter MJ, Pader P, Gall H, Reichenberger F, Seeger W, Mayer E, Guth S, Kramm T, Grimminger F, Ghofrani HA, Voswinckel R. The prognostic relevance of oxygen uptake in inoperable chronic thromboembolic pulmonary hypertension. Clin Respir J. 2017;11:682-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 114. | Iwase T, Nagaya N, Ando M, Satoh T, Sakamaki F, Kyotani S, Takaki H, Goto Y, Ohkita Y, Uematsu M, Nakanishi N, Miyatake K. Acute and chronic effects of surgical thromboendarterectomy on exercise capacity and ventilatory efficiency in patients with chronic thromboembolic pulmonary hypertension. Heart. 2001;86:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Fukui S, Ogo T, Goto Y, Ueda J, Tsuji A, Sanda Y, Kumasaka R, Arakawa T, Nakanishi M, Fukuda T, Takaki H, Yasuda S, Ogawa H, Nakanishi N. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015;180:66-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Jin Q, Luo Q, Yang T, Zeng Q, Yu X, Yan L, Zhang Y, Zhao Q, Ma X, An C, Xiong C, Zhao Z, Liu Z. Improved hemodynamics and cardiopulmonary function in patients with inoperable chronic thromboembolic pulmonary hypertension after balloon pulmonary angioplasty. Respir Res. 2019;20:250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Inagaki T, Terada J, Tanabe N, Kawata N, Kasai H, Sugiura T, Shigeta A, Asano Y, Murata A, Tsushima K, Tada Y, Sakao S, Tatsumi K. Home-based pulmonary rehabilitation in patients with inoperable or residual chronic thromboembolic pulmonary hypertension: a preliminary study. Respir Investig. 2014;52:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Zabini D, Heinemann A, Foris V, Nagaraj C, Nierlich P, Bálint Z, Kwapiszewska G, Lang IM, Klepetko W, Olschewski H, Olschewski A. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2014;44:951-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 119. | Magon W, Stepniewski J, Jonas K, Waligora M, Podolec P, Kopec G. P4679Changes in systemic inflammation and endothelial dysfunction after balloon pulmonary angioplasty. Eur Heart J. 2019;40:ehz745.1061. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 120. | Moser B, Megerle A, Bekos C, Janik S, Szerafin T, Birner P, Schiefer AI, Mildner M, Lang I, Skoro-Sajer N, Sadushi-Kolici R, Taghavi S, Klepetko W, Ankersmit HJ. Local and systemic RAGE axis changes in pulmonary hypertension: CTEPH and iPAH. PLoS One. 2014;9:e106440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Suzuki S, Nakazato K, Sugimoto K, Yoshihisa A, Yamaki T, Kunii H, Suzuki H, Saitoh S, Takeishi Y. Plasma Levels of Receptor for Advanced Glycation End-Products and High-Mobility Group Box 1 in Patients With Pulmonary Hypertension. Int Heart J. 2016;57:234-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Kamimura Y, Okumura N, Adachi S, Shimokata S, Tajima F, Nakano Y, Hirashiki A, Murohara T, Kondo T. Usefulness of scoring right ventricular function for assessment of prognostic factors in patients with chronic thromboembolic pulmonary hypertension. Heart Vessels. 2018;33:1220-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Sato H, Ota H, Sugimura K, Aoki T, Tatebe S, Miura M, Yamamoto S, Yaoita N, Suzuki H, Satoh K, Takase K, Shimokawa H. Balloon Pulmonary Angioplasty Improves Biventricular Functions and Pulmonary Flow in Chronic Thromboembolic Pulmonary Hypertension. Circ J. 2016;80:1470-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 124. | Yamada N. Beneficial Therapeutic Effects of Balloon Pulmonary Angioplasty on Biventricular Function in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ J. 2016;80:1326-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Kanar BG, Mutlu B, Atas H, Akaslan D, Yıldızeli B. Improvements of right ventricular function and hemodynamics after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Echocardiography. 2019;36:2050-2056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Sumimoto K, Tanaka H, Mukai J, Yamashita K, Tanaka Y, Shono A, Suzuki M, Yokota S, Suto M, Takada H, Matsumoto K, Taniguchi Y, Emoto N, Hirata KI. Effects of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension on remodeling in right-sided heart. Int J Cardiovasc Imaging. 2020;36:1053-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Broch K, Murbraech K, Ragnarsson A, Gude E, Andersen R, Fiane AE, Andreassen J, Aakhus S, Andreassen AK. Echocardiographic evidence of right ventricular functional improvement after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35:80-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 128. | Kimura M, Kataoka M, Kawakami T, Inohara T, Takei M, Fukuda K. Balloon pulmonary angioplasty using contrast agents improves impaired renal function in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015;188:41-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Darocha S, Banaszkiewicz M, Pietrasik A, Siennicka A, Piorunek M, Grochowska E, Piłka M, Dobosiewicz A, Florczyk M, Pietura R, Torbicki A, Kurzyna M. Changes in Estimated Glomerular Filtration after Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Cardiorenal Med. 2020;10:22-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Kriechbaum SD, Wiedenroth CB, Hesse ML, Ajnwojner R, Keller T, Sebastian Wolter J, Haas M, Roller FC, Breithecker A, Rieth AJ, Guth S, Rolf A, Hamm CW, Mayer E, Liebetrau C. Development of renal function during staged balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Scand J Clin Lab Invest. 2019;79:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Tatebe S, Sugimura K, Aoki T, Miura M, Nochioka K, Yaoita N, Suzuki H, Sato H, Yamamoto S, Satoh K, Fukumoto Y, Shimokawa H. Multiple Beneficial Effects of Balloon Pulmonary Angioplasty in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ J. 2016;80:980-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | Aoki T, Sugimura K, Tatebe S, Miura M, Yamamoto S, Yaoita N, Suzuki H, Sato H, Kozu K, Konno R, Miyata S, Nochioka K, Satoh K, Shimokawa H. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J. 2017;38:3152-3159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 133. | Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, Treacy C, Ponnaberanam A, Condliffe R, Sheares K, Taboada D, Dunning J, Tsui S, Ng C, Gopalan D, Screaton N, Elliot C, Gibbs S, Howard L, Corris P, Lordan J, Johnson M, Peacock A, MacKenzie-Ross R, Schreiber B, Coghlan G, Dimopoulos K, Wort SJ, Gaine S, Moledina S, Jenkins DP, Pepke-Zaba J. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation. 2016;133:1761-1771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 134. | Moriyama K, Satoh T, Motoyasu A, Kohyama T, Kotani M, Kanai R, Ando T, Yorozu T. High-Flow Nasal Cannula Therapy in a Patient with Reperfusion Pulmonary Edema following Percutaneous Transluminal Pulmonary Angioplasty. Case Rep Pulmonol. 2014;2014:837612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 135. | Adams A, Fedullo PF. Postoperative management of the patient undergoing pulmonary endarterectomy. Semin Thorac Cardiovasc Surg. 2006;18:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14:274-282. [PubMed] [Cited in This Article: ] |
| 137. | Sugiyama K, Suzuki S, Fujiyoshi T, Koizumi N, Sato M, Ogino H. Extracorporeal membrane oxygenation after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Card Surg. 2019;34:428-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 138. | Hosokawa K, Yamamoto T, Abe K, Tsutsui H. Delayed-onset lung injury after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension-a case report. Eur Heart J Cardiovasc Imaging. 2017;18:1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Ejiri K, Ogawa A, Matsubara H. Bail-out technique for pulmonary artery rupture with a covered stent in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2015;8:752-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 140. | Araszkiewicz A, Jankiewicz S, Zabicki B, Grygier M, Lesiak M. Rescue Implantation of Covered Stent in Pulmonary Artery Rupture During Balloon Pulmonary Angioplasty. J Invasive Cardiol. 2018;30:E122-E123. [PubMed] [Cited in This Article: ] |
| 141. | D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2500] [Cited by in F6Publishing: 2339] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 142. | Humbert M, Sitbon O, Yaïci A, Montani D, O'Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, Chaouat A, Chabot F, Cordier JF, Habib G, Gressin V, Jing ZC, Souza R, Simonneau G; French Pulmonary Arterial Hypertension Network. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 143. | Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24:272-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 144. | Bonderman D, Nowotny R, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Klepetko W, Lang IM. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest. 2005;128:2599-2603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 145. | Reichenberger F, Voswinckel R, Enke B, Rutsch M, El Fechtali E, Schmehl T, Olschewski H, Schermuly R, Weissmann N, Ghofrani HA, Grimminger F, Mayer E, Seeger W. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2007;30:922-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 146. | Sadushi-Kolici R, Lang IM. Treprostinil for the treatment of chronic thromboembolic pulmonary hypertension. Expert Rev Respir Med. 2019;1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 147. | Wiedenroth CB, Ghofrani HA, Adameit MSD, Breithecker A, Haas M, Kriechbaum S, Rieth A, Hamm CW, Mayer E, Guth S, Liebetrau C. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ. 2018;8:2045894018783996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 148. | Tsugu T, Murata M, Kawakami T, Kataoka M, Nagatomo Y, Tsuruta H, Itabashi Y, Maekawa Y, Fukuda K. Amelioration of right ventricular function after hybrid therapy with riociguat and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2016;221:227-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Wiedenroth CB, Liebetrau C, Breithecker A, Guth S, Lautze HJ, Ortmann E, Arlt M, Krombach GA, Bandorski D, Hamm CW, Möllmann H, Mayer E. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016;35:591-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 150. | Kawashima T, Yoshitake A, Kawakami T, Shimizu H. Two-stage Treatment Using Balloon Pulmonary Angioplasty and Pulmonary Endarterectomy in a Patient with Chronic Thromboembolic Pulmonary Hypertension. Ann Vasc Surg. 2018;49:315.e5-315.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | Danilov NM, Karabasheva MB, Sagaydak OV, Matchin YG, Mershin KV, Chazova IE. Hybrid approach for treating patient with chronic thromboembolic pulmonary hypertension and extrinsic compression of left main coronary artery. Rus OMJ. 2019;8:e0407. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 152. | Yanaka K, Nakayama K, Shinke T, Shinkura Y, Taniguchi Y, Kinutani H, Tamada N, Onishi H, Tsuboi Y, Satomi-Kobayashi S, Otake H, Tanaka H, Okita Y, Emoto N, Hirata KI. Sequential Hybrid Therapy With Pulmonary Endarterectomy and Additional Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J Am Heart Assoc. 2018;7:e008838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 153. | Kopeć G, Stępniewski J, Waligóra M, Kurzyna M, Biederman A, Podolec P. Staged treatment of central and peripheral lesions in chronic thromboembolic pulmonary hypertension. Pol Arch Med Wewn. 2016;126:97-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 154. | Kawakami T, Matsubara H, Abe K, Kataoka M, Kohsaka S, Sato Y, Shinke T, Fukuda K. Multicentre randomised controlled trial of balloon pulmonary angioplasty and riociguat in patients with chronic thromboembolic pulmonary hypertension: protocol for the MR BPA study. BMJ Open. 2020;10:e028831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 155. | Jais X, Brenot P, Bouvaist H, Canuet M, Chabanne C, Chaouat A, Cottin V, De Groote P, Dromer C, Favrolt N, Alonso CG, Gérardin B, Horeau-Langlard D, Jevnikar M, Magro P, Montani D, Parent F, Pison C, Prévot G, Renard S, Savale L, Simon AC, Sitbon O, Tresorier R, Tromeur C, Agostini H, Piedvache C, Fadel E, Humbert M, Simonneau G. Late Breaking Abstract - Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension: results from the randomised controlled RACE study. Eur Respir J. 2019;54:RCT1885. [DOI] [Cited in This Article: ] |
| 156. | Venkatesan P. 2019 European Respiratory Society International Congress. Lancet Respir Med. 2019;7:1007-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 157. | Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1236] [Cited by in F6Publishing: 1109] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 158. | Guérin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, Pontal D, Guégan M, Simonneau G, Meyer G, Sanchez O. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112:598-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 159. | Oh DK, Song JM, Park DW, Oh SY, Ryu JS, Lee J, Lee SD, Lee JS. The effect of a multidisciplinary team on the implementation rates of major diagnostic and therapeutic procedures of chronic thromboembolic pulmonary hypertension. Heart Lung. 2019;48:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 160. | Siennicka A, Darocha S, Banaszkiewicz M, Kędzierski P, Dobosiewicz A, Błaszczak P, Peregud-Pogorzelska M, Kasprzak JD, Tomaszewski M, Mroczek E, Zięba B, Karasek D, Ptaszyńska-Kopczyńska K, Mizia-Stec K, Mularek-Kubzdela T, Doboszyńska A, Lewicka E, Ruchała M, Lewandowski M, Łukasik S, Chrzanowski Ł, Zieliński D, Torbicki A, Kurzyna M. Treatment of chronic thromboembolic pulmonary hypertension in a multidisciplinary team. Ther Adv Respir Dis. 2019;13:1753466619891529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |