Copyright
©The Author(s) 2019.
World J Clin Cases. Dec 6, 2019; 7(23): 4036-4043
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4036
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4036
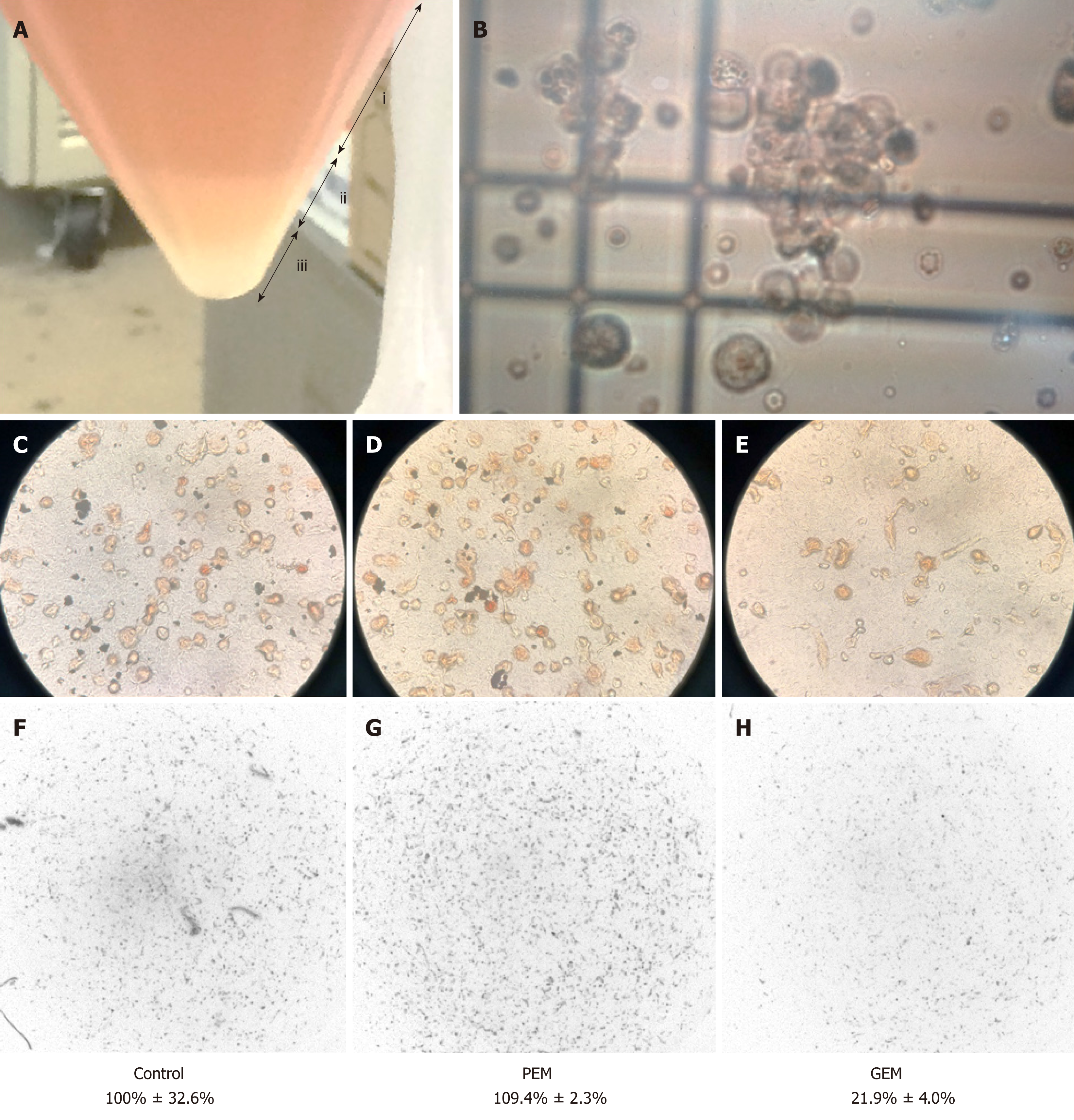
Figure 3 Collagen gel droplet-embedded culture drug sensitivity test assay of mesothelioma cells obtained from ascites.
A: The filtered cancer cells were centrifuged to divide the fluid into a supernatant (i), mucus component (ii), and cell pellet including mesothelioma cells (iii); B: After cell counting, the mesothelioma cells were used to conduct the collagen gel droplet-embedded culture drug sensitivity test (CD-DST). Tumor cells were incubated without any cytotoxic drug; C: Or in the presence of cytotoxic drugs such as D: pemetrexed (PEM) and E: gemcitabine . After the proliferation assay, the final number of cancer cells was quantified using a dedicated system; F: Compared to a normal control, PEM did not reduce cell growth, while gemcitabine reduced the tumor cell growth rate to 21.9%. PEM: Pemetrexed; GEM: Gemcitabine.
- Citation: Anayama T, Taguchi M, Tatenuma T, Okada H, Miyazaki R, Hirohashi K, Kume M, Matsusaki K, Orihashi K. In-vitro proliferation assay with recycled ascitic cancer cells in malignant pleural mesothelioma: A case report. World J Clin Cases 2019; 7(23): 4036-4043
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4036