Published online Sep 26, 2018. doi: 10.12998/wjcc.v6.i10.373
Peer-review started: June 8, 2018
First decision: July 3, 2018
Revised: July 17, 2018
Accepted: August 27, 2018
Article in press: August 28, 2018
Published online: September 26, 2018
Processing time: 110 Days and 18.8 Hours
To investigate the safety and efficacy of S-1 plus oxaliplatin (SOX) as an adjuvant chemotherapy regimen in gastric cancer (GC) after D2 dissection.
GC Patients who underwent D2 gastrectomy from September 2009 to December 2011 in four Chinese institutions were enrolled. Patients with stage IB-IIIC GC, who received adjuvant SOX treatment were matched by propensity scores with those who underwent surgery alone and those who conducted capecitabine plus oxaliplatin (XELOX) regimen. Disease-free survival (DFS) and overall survival (OS) were compared among the groups. In addition, adverse events in SOX patients were analyzed.
Of 1944 GC patients who underwent D2 dissection, 867 were included for analysis. One hundred and seventeen patients treated with SOX were matched to 234 patients who conducted surgery alone. Fifty-seven patients treated with SOX were matched to 57 patients who received XELOX. The estimated five-year DFS was 57.5% in the adjuvant SOX group which was higher than that (44.6%) in the surgery alone group (P = 0.001); and the estimated five-year OS was 68.3% which was higher than that (45.8%) of surgery alone group (P < 0.001). Survival benefit was also revealed in stage III and > 60 years old subgroups (P < 0.001 and P = 0.015, respectively). Compared with XELOX regimen, SOX showed no significant difference in DFS (P = 0.340) and OS (P = 0.361). The most common ≥ 3 grade adverse events of SOX regimen were neutropenia (22.6%), leukopenia (8.9%) and thrombocytopenia (5.6%).
Compared with surgery alone, SOX regimen significantly improves the long-term survival and has acceptable toxicity in patients with stage IB-IIIC GC after D2 dissection. It may be a novel adjuvant chemotherapy regimen in GC patients.
Core tip: Based on the therapeutic efficacy of both S-1 mono-therapy and oxaliplatin plus capecitabine regimen in ACTS-gastric cancer (GC) and CLASSIC study, we conducted the multi-institutional research using propensity score-matched analysis to evaluate whether patients after D2 resection benefit from adjuvant chemotherapy with S-1 plus oxaliplatin (SOX). Here, we firstly report that SOX adjuvant chemotherapy, compared with surgery alone, significantly improves disease-free survival and overall survival in stage IB-IIIC GC patients undergoing D2 resection with accepted side effects.
- Citation: Ren DF, Zheng FC, Zhao JH, Shen GS, Ahmad R, Zhang SS, Zhang Y, Kan J, Dong L, Wang ZY, Zhao FX, Zhao JD. Adjuvant chemotherapy with S-1 plus oxaliplatin improves survival of patients with gastric cancer after D2 gastrectomy: A multicenter propensity score-matched study. World J Clin Cases 2018; 6(10): 373-383
- URL: https://www.wjgnet.com/2307-8960/full/v6/i10/373.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i10.373
Gastric cancer (GC) is one of the most common malignancies with high morbidity and mortality worldwide[1]. Adequate surgical resection is the only curative therapeutic option for GC. In East Asia, gastrectomy with D2 lymphadenectomy is the standard surgical treatment[2,3]. In fact, based on the results of the Dutch D1D2 trial[4], the European and United States guidelines have likewise recommended the procedure[5,6]. However, even with a potentially curative resection, approximately 50% of patients develop recurrence within 5 years after surgery[7,8], and 50%-90% of patients die of tumor relapses[9].
To decrease the risk of postoperative recurrence, various regimens for adjuvant chemotherapy have been implemented over the past 40 years. Results of two large randomized phase 3 trials, which are the ACTS-GC and CLASSIC trials, have shown survival benefit from adjuvant chemotherapy in patients who underwent D2 radical resection for stage II-III disease[7,8]. In the ACTS-GC study, intake of S-1 treatment for one year after D2 gastrectomy increased the five-year relapse-free survival (RFS) and overall survival (OS) by 12.3% and 10.6%, respectively[7]. In CLASSIC trial, 6 mo of capecitabine plus oxaliplatin (XELOX) therapy improved the estimated five-year disease-free survival (DFS) and OS by 15% and 9%, respectively[8].
To date, only the two adjuvant chemotherapy regimens mentioned above have been proven to be significantly efficient in stage II-III GC patients who underwent D2 dissection. However, some aspects in the previous two studies on adjuvant chemotherapy need to be improved. In the ACTS-GC trial, patients had a low compliance (65.8%) in taking S-1 for one year and a subgroup analysis showed that the effect was insufficient in the elderly or stage III patients[7]. In the CLASSIC study, patients also had a low treatment completion rate (67%)[8]. Therefore, new adjuvant chemotherapy regimens need to be explored.
Considering that both S-1 mono-therapy and combination therapy with oxaliplatin plus capecitabine have become the standard treatment for GC patients after D2 gastrectomy, a phase 2, single-arm study that investigated the safety of adjuvant chemotherapy regimen with S-1 plus oxaliplatin (SOX) in Japanese patients showed better toxicity profiles and relatively high completion rate (74.2%)[10]. Therefore, adjuvant chemotherapy with SOX for GC is most likely reasonable and efficacious. Based on the aforementioned, we conducted this multicenter retrospective study to evaluate the safety and efficacy of SOX as adjuvant chemotherapy in stage IB-IIIC GC after D2 gastrectomy.
The study included GC patients who underwent D2 gastrectomy at the Affiliated Hospital of Qinghai University, People’s Hospital of Qinghai Province, Qinghai Red Cross Hospital, and Cancer Institute and Hospital, Chinese Academy of Medical Sciences from September 2009 to December 2011. Patients were selected if they met the following eligibility criteria: (1) histologically confirmed adenocarcinoma of the stomach; and (2) stage IB (pT1N1M0) or IB (pT2N0M0) with high-risk features including poorly differentiated or higher grade cancer, lymphovascular invasion, neural invasion, or < 50 years of age, stage II, or III disease according to the Seventh Edition of the American Joint Committee on Cancer (AJCC) classification. The following patients were excluded: (1) stage IA or IB (pT2N0M0) disease without aforementioned high-risk features; (2) those who received radiotherapy before or after surgery; (3) those who received neo-adjuvant chemotherapy; and (4) those who received adjuvant chemotherapy except for SOX or XELOX regimens.
We analyzed the clinicopathologic characteristics of the enrolled patients, including age, sex, tumor location, tumor grade, p-TNM stage (based on the Seventh AJCC classification), lymphatic and venous invasion, and perineural invasion. All eligible patients were divided into three parts, patients who treated with surgery alone, patients who received postoperative SOX adjuvant chemotherapy, and those who received XELOX adjuvant chemotherapy.
The study was approved by the institutional review boards of the Affiliated Hospital of Qinghai University, People’s Hospital of Qinghai Province, Qinghai Red Cross Hospital, and Cancer Institute and Hospital, Chinese Academy of Medical Sciences.
SOX adjuvant chemotherapy was started within 3-6 wk after D2 gastrectomy. In all 3-wk cycles, S-1 was given orally twice daily for 2 wk at a dose of 80 mg/d for patients with a body surface area (BSA) < 1.25 m2, 100 mg/d for patients with a BSA of 1.25 m2 to < 1.5 m2, and 120 mg/d for patients with a BSA of ≥ 1.5 m2. On day 1 of each chemotherapy cycle, oxaliplatin was infused intravenously for 2-4 h at a dose of 130 mg/m2. The Common Toxicity Criteria of the National Cancer Institute (version 4.0) was used to assess the adverse effects of chemotherapy. XELOX adjuvant chemotherapy was also started within 3-6 wk after D2 dissection. Capecitabine was given orally at a dose of 1000 mg/m2 twice daily on days 1 to 14 of each 3-wk cycle. Oxaliplatin at 130 mg/m2 was infused intravenously for 2-4 h on day 1 of each chemotherapy cycle.
Patients in the surgery alone group did not receive any antineoplastic agent until there was a confirmed recurrence. All the enrolled patients underwent hematologic tests, physical examination, and computed tomography every three months for the first two years after surgery, every six months from the third year to the fifth year, and annually thereafter.
Data, including tumor relapse, death from any cause and the last follow-up date were collected. DFS was defined as the time from surgery to tumor recurrence or the last follow-up date. OS was defined as the time from surgery to death or the last follow-up date.
To compare the baseline clinicopathologic characteristics between the adjuvant SOX and the surgery alone groups, the adjuvant SOX and the adjuvant XELOX groups, the χ2 test or Fisher’s exact test was used for categorical variables, whereas the Mann-Whitney U test was used for continuous variables. Survival outcomes were estimated using the Kaplan-Meier method, and the differences in survival between the treatment groups were compared using the log-rank test. An unadjusted Cox proportional hazards model was used to calculate the hazard ratio (HR) with the 95% confidence interval (CI) for the survival outcomes in all groups. To determine the independent prognostic factors for OS, a multiple regression analysis using a Cox proportional hazards model was performed. All tests were two-sided, and P < 0.05 was considered statistically significant.
We used propensity score matching to reduce to the greatest extent the effects of selection bias and the possible confounding factors. Propensity scores were estimated by a logistic regression model of the following covariates: age, sex, tumor location, tumor grade, p-TNM stage, lymphatic and venous invasion, and perineural invasion. Patients in the adjuvant SOX group were matched in a 1:2 ration with those in the surgery alone group and 1:1 ration with those in the adjuvant XELOX group using calculated propensity scores with a 0.05 caliper width. And only the patients matched with propensity scores were included in the time-to-event analyses. We performed the propensity score matching using the Matching package in R, version 3.3.1 (R Foundation)[11].
Sensitivity analysis was conducted by adding co-morbidity to our propensity score model before repeating the DFS and OS analyses between the adjuvant SOX group and the surgery alone group. Except for the propensity score matching, all statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, IL, United States).
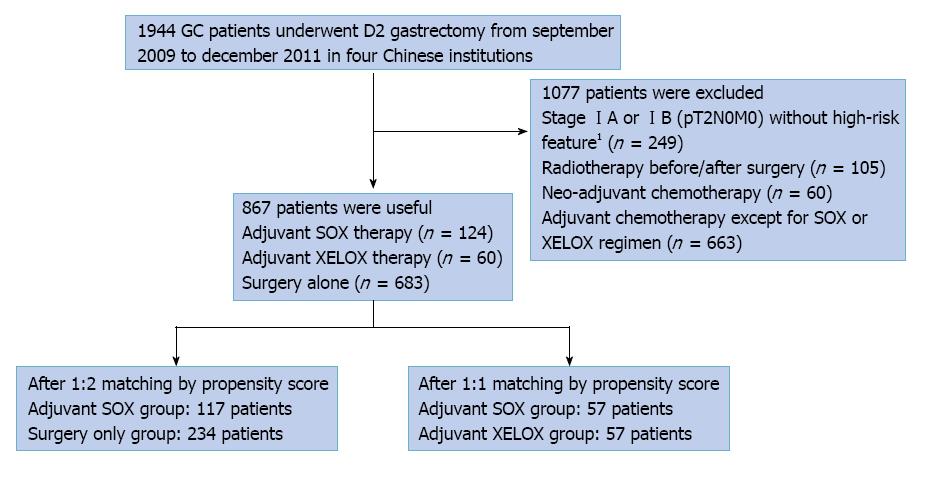
From September 2009 to December 2011, there were 1944 GC patients who were treated by curative gastrectomy with D2 lymphadenectomy. Among them, 1077 patients were excluded for being in stage IA or IB (pT2N0M0) without high-risk features (n = 249), having received radiotherapy before or after surgery (n = 105), neo-adjuvant chemotherapy (n = 60), other adjuvant chemotherapy except for SOX or XELOX regimens after surgery (n = 663). A total of 867 patients were analyzed in this study; 124 patients received SOX adjuvant chemotherapy, 60 patients received XELOX adjuvant therapy and 683 patients underwent surgery alone. After propensity score matching, 117 pairs of 1:2 matched patients (i.e., 351patients) and 57 pairs of 1:1 matched patients (i.e., 114 patients) were generated (Figure 1).
The clinicopathologic characteristics of patients in adjuvant SOX group and surgery alone group before and after matching are shown in Table 1. Overall, compared with the surgery alone group, the adjuvant SOX group had more poor to moderate grade tumor (78.23% vs 62.52%), more pathologic stage III cancer (70.16% vs 37.19%), and more lymphatic and venous invasion (30.65% vs 16.11%). After matching, all the baseline clinicopathologic characteristics including age, sex, tumor location, tumor grade, p-TNM stage, lymphatic and venous invasion, and perineural invasion, were similar between the two groups. The clinicopathologic characteristics of patients in adjuvant SOX group and adjuvant XELOX group before and after matching are shown in Table 2.
| Variable | Before PSM (n = 807) | After PSM (n = 351) | ||||
| Surgery alone(n = 683) | Adjuvant SOX(n = 124) | P value | Surgery alone(n = 234) | Adjuvant SOX(n = 117) | P value | |
| Age at diagnosis (yr) | 0.099 | 0.443 | ||||
| < 35 | 9 (1.32) | 3 (2.42) | 3 (1.28) | 0 (0.00) | ||
| 35-60 | 303 (44.36) | 66 (53.23) | 118 (50.43) | 62 (52.99) | ||
| > 60 | 371 (54.32) | 55 (44.35) | 113 (48.29) | 55 (47.01) | ||
| Gender | 0.83 | 1 | ||||
| Female | 177 (25.92) | 31 (25.00) | 60 (25.64) | 30 (25.64) | ||
| Male | 506 (74.08) | 93 (75.00) | 174 (74.36) | 87 (74.36) | ||
| Tumor location | 0.063 | 0.424 | ||||
| None-Cardia cancer | 425 (62.23) | 88 (70.97) | 152 (64.96) | 81 (69.23) | ||
| Cardia cancer | 258 (37.77) | 36 (29.03) | 82 (35.04) | 36 (30.77) | ||
| Tumor grade | 0.006 | 0.725 | ||||
| Moderate to well | 50 (7.32) | 4 (3.23) | 14 (5.98) | 4 (3.42) | ||
| Moderate | 171 (25.04) | 21 (16.94) | 36 (15.38) | 21 (17.95) | ||
| Poor to moderate | 427 (62.52) | 97 (78.23) | 180 (76.92) | 90 (76.92) | ||
| Early cancer or not reported | 35 (5.12) | 2 (1.61) | 4 (1.71) | 2 (1.71) | ||
| Pathological stage | < 0.001 | 0.604 | ||||
| IB1 | 302 (44.22) | 8 (6.45) | 20 (8.55) | 8 (6.84) | ||
| II | 127 (18.59) | 29 (23.39) | 48 (20.51) | 29 (24.79) | ||
| III | 254 (37.19) | 87 (70.16) | 166 (70.94) | 80 (68.38) | ||
| Lymphatic and venous invasion | < 0.001 | 0.574 | ||||
| No | 573 (83.89) | 86 (69.35) | 155 (66.24) | 81 (69.23) | ||
| Yes | 110 (16.11) | 38 (30.65) | 79 (33.76) | 36 (30.77) | ||
| Perineural invasion | 0.437 | 0.754 | ||||
| No | 606 (88.73) | 107 (86.29) | 197 (84.19) | 100 (85.47) | ||
| Yes | 77 (11.27) | 17 (13.71) | 37 (15.81) | 17 (14.53) | ||
| Variable | Before PSM (n = 184) | After PSM (n = 114) | ||||
| Adjuvant SOX(n = 124) | Adjuvant XELOX(n = 60) | P value | Adjuvant SOX(n = 57) | Adjuvant XELOX(n = 57) | P value | |
| Age at diagnosis (yr) | 0.322 | 0.848 | ||||
| ≤ 60 | 69 (55.65) | 38 (63.33) | 34 (59.65) | 35 (61.40) | ||
| > 60 | 55 (44.35) | 22 (36.67) | 23 (40.35) | 22 (38.60) | ||
| Gender | 1 | 0.404 | ||||
| Female | 31 (25.00) | 15 (25.00) | 18 (31.58) | 14 (24.56) | ||
| Male | 93 (75.00) | 45 (75.00) | 39 (68.42) | 43 (75.44) | ||
| Tumor location | 0.567 | 1 | ||||
| None-Cardia cancer | 88 (70.97) | 45 (75.00) | 42 (73.68) | 42 (73.68) | ||
| Cardia cancer | 36 (29.03) | 15 (25.00) | 15 (26.32) | 15 (26.32) | ||
| Tumor grade | 0.521 | 0.233 | ||||
| Moderate to well | 4 (3.23) | 1 (1.67) | 4 (7.02) | 1 (1.75) | ||
| Moderate | 21 (16.94) | 14 (23.33) | 13 (22.81) | 12 (21.05) | ||
| Poor to moderate | 97 (78.23) | 45 (75.00) | 38 (66.67) | 44 (77.19) | ||
| Early cancer or not reported | 2 (1.61) | 0 (0.00) | 2 (3.51) | 0 (0.00) | ||
| Pathological stage | 0.038 | 0.708 | ||||
| IB1 | 8 (6.45) | 11 (18.33) | 7 (12.28) | 9 (15.79) | ||
| II | 29 (23.39) | 10 (16.67) | 12 (21.05) | 9 (15.79) | ||
| III | 87 (70.16) | 39 (65.00) | 38 (66.67) | 39 (68.42) | ||
| Lymphatic and venous invasion | 0.929 | 0.839 | ||||
| No | 86 (69.35) | 42 (70.00) | 39 (68.42) | 40 (70.18) | ||
| Yes | 38 (30.65) | 18 (30.00) | 18 (31.58) | 17 (29.82) | ||
| Perineural invasion | 0.102 | 1 | ||||
| No | 107 (86.29) | 46 (76.67) | 46 (80.70) | 46 (80.70) | ||
| Yes | 17 (13.71) | 14 (23.33) | 11 (19.30) | 11 (19.30) | ||
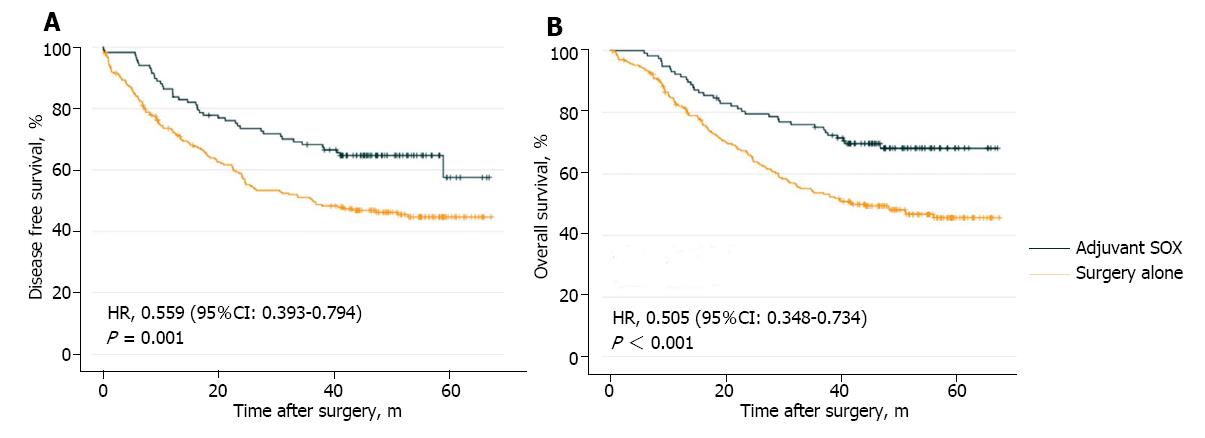
In adjuvant SOX group, a median cycle of 4 (1-12 cycles) were received. After a median follow-up of 42 mo after gastrectomy, the number of patients who developed relapse and died was 42 (35.9%) and 36 (30.8%), respectively, in the adjuvant SOX group and 122 (52.1%) and 117 (50.0%), respectively, in the surgery alone group. The estimated five-year DFS was 57.5% in the adjuvant SOX group and 44.6% in the surgery alone group (HR = 0.559; 95%CI: 0.393-0.794; P = 0.001; Figure 2A). The estimated five-year OS was 68.3% in the adjuvant SOX group and 45.8% in the surgery alone group (HR = 0.505; 95%CI: 0.348-0.734; P < 0.001; Figure 2B).
After addition of co-morbidity to the propensity score model in the sensitivity analysis, 116 pairs of 1:2 matched patients (i.e., 348 patients) were generated. Repeat analyses showed that compared with the surgery alone group, the adjuvant SOX group had significantly better DFS (HR = 0.542; 95%CI: 0.377-0.779; P = 0.001) and OS (HR = 0.496; 95%CI: 0.338-0.728; P < 0.001).
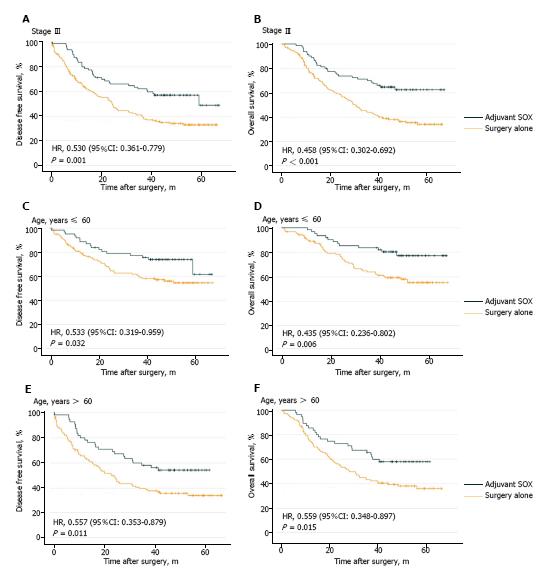
To further investigate whether stage III or elderly patients can benefit from SOX adjuvant chemotherapy, exploratory subgroup analyses were performed among the 351 matched patients. In the stage III patients, the estimated five-year DFS rates were 33.4% in the surgery alone group and 49.1% in the adjuvant SOX group, with an HR of 0.530 (95%CI: 0.361-0.779; P = 0.001; Figure 3A). The estimated five-year OS rates were 34.1% in the surgery alone group and 62.5% in the adjuvant SOX group, with an HR of 0.458 (95%CI: 0.302-0.692; P < 0.001; Figure 3B).
In patients aged ≤ 60 years, the estimated five-year DFS rates were 54.5% in the surgery alone group and 61.6% in the adjuvant SOX group, with an HR of 0.553 (95%CI: 0.319-0.959; P = 0.032; Figure 3C). The estimated five-year OS rates were 55.2% in the surgery alone group and 77.2% in the adjuvant SOX group, with an HR of 0.435 (95%CI: 0.236–0.802; P = 0.006; Figure 3D).
For patients > 60 years old, the estimated five-year DFS rates were 34.2% in the surgery alone group and 54.4% in the adjuvant SOX group, with an HR of 0.557 (95%CI: 0.353-0.879; P = 0.011; Figure 3E). The estimated five-year OS rates were 36.0% in the surgery alone group and 58.0% in the adjuvant SOX group, with an HR of 0.559 (95%CI: 0.348-0.897; P = 0.015; Figure 3F).
The multivariate Cox proportional hazards model showed that age (HR, 1.629; 95%CI: 1.155-2.297; P = 0.005), p-TNM stage III (HR = 10.258; 95%CI: 2.202-47.783; P = 0.003), perineural invasion (HR = 1.637; 95%CI: 1.056-2.538; P = 0.028), and SOX adjuvant chemotherapy (HR = 0.481; 95%CI: 0.329-0.702; P < 0.001) were the independent prognostic factors for OS of GC patients after D2 gastrectomy (Table 3).
| Variable | Hazard ratio | 95%CI | P value |
| Age at diagnosis (yr) | |||
| ≤ 60 | 1 | ||
| > 60 | 1.629 | (1.155-2.297) | 0.005 |
| Gender | |||
| Female | 1 | ||
| Male | 1.073 | (0.731-1.576) | 0.718 |
| Tumor location | |||
| None-Cardia cancer | 1 | ||
| Cardia cancer | 0.862 | (0.605-1.227) | 0.409 |
| Tumor grade | 0.888 | ||
| Moderate to well | 1 | ||
| Moderate | 1.37 | (0.558-3.363) | 0.492 |
| Poor to moderate | 1.283 | (0.551-2.983) | 0.563 |
| Early cancer or not reported | 1.972 | (0.205-18.948) | 0.557 |
| Pathological stage | < 0.001 | ||
| IB1 | 1 | ||
| II | 4.691 | (0.994-22.128) | 0.051 |
| III | 9.857 | (2.174-44.686) | 0.003 |
| Lymphatic and venous invasion | |||
| No | 1 | ||
| Yes | 0.963 | (0.670-1.384) | 0.837 |
| Perineural invasion | |||
| No | 1 | ||
| Yes | 1.679 | (1.091-2.585) | 0.019 |
| Adjuvant chemotherapy | |||
| Surgery alone | 1 | ||
| SOX | 0.475 | (0.326-0.693) | < 0.001 |
Out of 124 patients who received SOX adjuvant chemotherapy, 122 patients (98.4%) developed different grades of adverse events. Table 4 shows all the grades of adverse events reported by ≥ 10% of patients. Grade 3 or 4 adverse events were reported by 47 (37.9%) patients, and the most common adverse events were neutropenia (22.6%), leukopenia (8.9%) and thrombocytopenia (5.6%). Among all grades of adverse events, neutropenia (75.0%), leukopenia (60.5%) and peripheral sensory neuropathy (52.4%) had the highest event rate. In addition, one patient developed febrile neutropenia during the chemotherapy period. Fifty-six patients (45.2%) experienced reduction of S-1 or/and oxaliplatin dose mainly because of the adverse events of neutropenia, leukopenia and peripheral sensory neuropathy. Seventy-eight patients (62.9%) had a delay in the subsequent treatment and the most common reason is the adverse event of neutropenia.
| Event | Adjuvant SOX (n = 124) | |||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | All grade | Grade 3 or 4 | |
| No. of patients | % | |||||
| Leukopenia | 46 | 18 | 8 | 3 | 60.5 | 8.9 |
| Neutropenia | 34 | 31 | 19 | 9 | 75 | 22.6 |
| Anemia | 35 | 3 | 1 | 1 | 32.3 | 1.6 |
| Thrombocytopenia | 22 | 18 | 7 | 0 | 37.9 | 5.6 |
| Elevated total serum bilirubin level | 23 | 2 | 1 | 0 | 21 | 0.8 |
| Elevated AST/ALT level | 53 | 5 | 1 | 1 | 48.4 | 1.6 |
| Elevated ALP level | 12 | 3 | 1 | 0 | 12.9 | 0.8 |
| Nausea | 41 | 19 | 4 | - | 51.6 | 3.2 |
| Vomiting | 17 | 16 | 4 | 0 | 29.8 | 3.2 |
| Diarrhea | 33 | 7 | 1 | 0 | 33.1 | 0.8 |
| Peripheral sensory neuropathy | 61 | 4 | 0 | - | 52.4 | 0 |
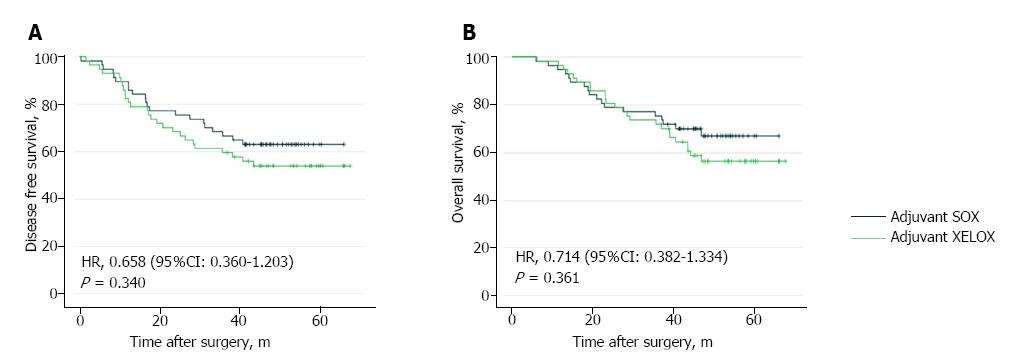
In adjuvant SOX group (57 patients), patients received 250 cycles chemotherapy in total with median cycles of 4. In adjuvant XELOX group (57 patients), patients received 258 cycles chemotherapy in total with median cycles of 5. After a median follow-up of 42 mo after gastrectomy, 47 patients developed relapse (21 in the SOX group and 26 in the XELOX group), 42 patients died (18 in the SOX group and 24 in the XELOX group). The estimated five-year DFS was 63.1% in the adjuvant SOX group and 54.0% in the adjuvant XELOX group (HR = 0.658; 95%CI: 0.360-1.203; P = 0.340; Figure 4A). The estimated five-year OS was 67.0% in the adjuvant SOX group and 56.5% in the adjuvant XELOX group (HR = 0.714; 95%CI: 0.382-1.334; P = 0.361; Figure 4B).
To the best of our knowledge, this was the first study to report that adjuvant chemotherapy with SOX can significantly improve the long-term survival of patients with GC after D2 radical gastrectomy, compared with surgery alone. After adjustment for confounders in the propensity score-matched analysis, adjuvant chemotherapy with SOX, compared with surgery alone, improved the estimated five-year DFS and OS by approximately 12.9% (P = 0.001) and 22.5% (P < 0.001), respectively, with mild and well-tolerated toxicities. The results were similar in the sensitivity analysis after addition of co-morbidity to the propensity score model; the estimated five-year DFS and OS improved by 13.9% (P = 0.001) and 22.2% (P < 0.001), respectively. Moreover, our research showed that SOX regimen was as effective as XELOX for stage IB-IIIC GC patients after D2 dissection. These results strongly suggest that SOX is very likely to become a novel adjuvant chemotherapy regimen in patients with GC after D2 radical resection.
D2 gastrectomy is the standard of surgical procedure in patients with GC in East Asia[12,13]. Moreover, the European and United States treatment guidelines have suggested such procedure in resectable patients, based on the Dutch D1D2 clinical study, which showed that D2 gastrectomy reduced the number of cancer-related deaths compared with D1[4-6,14,15]. Two recent, excellent, and large-scale randomized trials have shown that adjuvant chemotherapy can improve both DFS and OS in patients with resectable GC after D2 gastrectomy[7,8].
The ACTS-GC trial revealed that one year of adjuvant chemotherapy with S-1 for stage II/III GC patients after D2 dissection increased the five-year RFS and OS rates from 53.1% to 65.4% and 61.1% to 71.7%, respectively[7]. The phase 3 CLASSIC study reported that six months of adjuvant chemotherapy with capecitabine and oxaliplatin after curative D2 gastrectomy in stage II to IIIB GC patients improved the estimated five-year DFS and OS rates from 53% to 68% and 69% to 78%, respectively[8]. However, it should be noted that in the ACTS-GC study, the effect of adjuvant chemotherapy with S-1 in GC was stage-dependent. In particular, a superior treatment effect was observed in stage II cases (HR = 0.509), but it was rather ineffective for stage IIIA (HR = 0.708) and stage IIIB (HR = 0.791) disease[7]. These results suggested that S-1 treatment was insufficient in eliminating micrometastatic cancer cells in cases with high p-TNM stage. Furthermore, in their subgroup analysis, S-1 treatment was shown to be not beneficial in elderly patients (≥ 60 years) and could not be sustained up to one year, with a 12-mo completion rate of only 65.8%[16]. In the CLASSIC study, only 67% received the planned eight cycles of adjuvant capecitabine and oxaliplatin chemotherapy; 56% experienced grade 3 or 4 adverse events; and 90% needed dose modifications because of adverse events[17]. Therefore, novel adjuvant chemotherapy regimen with high efficiency and mild side effect needs to be explored for GC patients undergoing D2 dissection.
In this study, patients who received adjuvant chemotherapy with SOX had significantly better survival than those who underwent surgery alone. In the ACTS-GC trial and CLASSIC studies, both S-1 mono-therapy and oxaliplatin combined with capecitabine were confirmed to have a survival benefit for patients with GC after D2 dissection[7,8]. Moreover, SOX was shown to have a high response rate (55.7%) and disease control rate (85.2%) in advanced GC[18]. Therefore, adjuvant chemotherapy with SOX is reasonable for GC. A single-arm, phase 2 study revealed that adjuvant SOX treatment was manageable and safe with optimal dose reduction or delay in the initiation of a subsequent cycle in stage III GC patients undergoing D2 or more extensive lymphadenectomy[10]. Most recently, Wang et al[19] reported DFS (75.9%) and OS (85.2%) for 3 years by adjuvant SOX chemotherapy for Chinese patients in GC. However, there had been no study that evaluated the survival benefit of adjuvant SOX chemotherapy over surgery alone in GC patients after D2 gastrectomy.
In this study, the survival rates of propensity score-matched patients were compared between adjuvant SOX chemotherapy and surgery alone, adjuvant SOX and XELOX chemotherapy. The results showed that compared with surgery alone, adjuvant SOX chemotherapy had survival benefit in terms of DFS and OS. The 12.9% estimated five-year DFS benefit rate in this study was almost similar to the results of the ACTS-GC trial and CLASSIC studies. In this present study, the 22.5% significant improvement in the estimated five-year OS with SOX regimen was probably related to the adjuvant chemotherapy itself and to the fact that some patients with relapse in the surgery alone group declined further antineoplastic therapy, whereas the patients in the adjuvant chemotherapy group remained to receive palliative therapy after relapse or due to more other comorbidities or competing causes of death in surgery alone patients. Our exploratory subgroup analysis showed the same survival benefits of adjuvant SOX chemotherapy in stage III and elderly patients. These results were similar to those of the CLASSIC study, but were not consistent with those of the ACTS-GC clinical trial. These differences might suggest that adjuvant chemotherapy with a doublet regimen containing S-1 was superior to mono-therapy with S-1 in these patients.
In this study, the adverse events documented with SOX were similar with the reported safety profiles of SOX in a phase 2 adjuvant therapy study and a phase 3 palliative treatment study on GC[10,18]. The most common adverse events were neutropenia, leukopenia, nausea, peripheral sensory neuropathy, and mild elevation of hepatic transaminases. Overall, the frequency of adverse events ≥ grade 3 was less than 40%, suggesting that adjuvant SOX chemotherapy for GC after D2 radical gastrectomy was well tolerated.
The present study had several limitations. First, the baseline characteristics of the patients were different between both groups. Although we performed propensity score-matched analysis and multivariate regression to reduce biases, remnant heterogeneity between groups cannot be excluded. Second, although the entire study population was relatively large, the sample size of patients receiving adjuvant SOX or XELOX chemotherapy was not adequate for subgroup analyses according to each variable. Third, a majority of GC patients after D2 dissection in China were stage III disease which can’t benefit from S-1 monotherapy according to ACTS-GC trial; most of the patients in our study didn’t receive S-1 monotherapy. We didn’t analyze data about patients only receiving S-1 in our study. Forth, the definite relapse locations were not clear for part of the patients in our study, we didn’t analyse the data about site of first relapse between patients received SOX or XELOX chemotherapy and those underwent surgery alone. Fifth, considering this study comprised Chinese patients, the dose of adjuvant SOX in other populations, especially Caucasians, remains to be further investigated because of the differences in the pharmacokinetics and toxicities of S-1 between Caucasian and Asian patients[20]. Moreover, the role of SOX in patients undergoing D1 dissection needs to be confirmed.
In conclusion, compared with surgery alone, adjuvant SOX regimen significantly improved the long-term survival of Chinese patients with stage IB-IIIC GC after D2 radical gastrectomy, with accepted side effects. It showed the similar DFS and OS outcomes with XELOX regimen which had become the standard adjuvant therapy nowadays. Therefore, SOX is likely to become a novel adjuvant chemotherapy regimen in GC. Several ongoing studies on the role of SOX for adjuvant chemotherapy in GC are expected to convey new and definite proofs in future[21-24].
The main objectives of this retrospective study were to evaluate the safety and efficacy of S-1 plus oxaliplatin (SOX) as adjuvant chemotherapy for gastric cancer (GC) after D2 dissection.
We collected patients with GC who underwent D2 gastrectomy from September 2009 to December 2011 in four Chinese institutions. Patients with stage IB-IIIC GC, who received adjuvant SOX treatment were matched by propensity scores with those who underwent surgery alone and those who conducted adjuvant capecitabine plus oxaliplatin (XELOX) regimen. We compared the estimated 5-year disease-free survival (DFS) and 5-year overall survival (OS) between the groups and analyzed adverse events in SOX patients.
In total, 867 GC patients were included for analysis. Among 124 patients treated with SOX regimen, 117 patients were matched to 234 patients who conducted surgery alone, and 57 patients were matched to 57 patients who received XELOX regimen. The estimated five-year DFS was 57.5% in the adjuvant SOX group and 44.6% in the surgery alone group (P = 0.001); and the estimated five-year OS was 68.3% and 45.8% (P < 0.001), respectively. Compared with XELOX regimen, SOX showed no significant difference in DFS and OS. The most common ≥ 3 grade adverse events of SOX regimen were neutropenia (22.6%), leukopenia (8.9%) and thrombocytopenia (5.6%).
This study showed that compared with surgery alone, adjuvant SOX regimen significantly improves the long-term survival and have acceptable toxicity in patients with stage IB-IIIC GC after D2 dissection.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hashimoto N, Noshiro H S- Editor: Dou Y L- Editor: A E- Editor: Wu YXJ
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 21190] [Article Influence: 2119.0] [Reference Citation Analysis (2)] |
| 2. | Park JM, Kim YH. Current approaches to gastric cancer in Korea. Gastrointest Cancer Res. 2008;2:137-144. [PubMed] [Cited in This Article: ] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1874] [Article Influence: 234.3] [Reference Citation Analysis (1)] |
| 4. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1140] [Cited by in F6Publishing: 1280] [Article Influence: 85.3] [Reference Citation Analysis (1)] |
| 5. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1090] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 6. | National Comprehensive Cancer Network. Gastric Cancer (Version 5. 2017). Available from: http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. [Cited in This Article: ] |
| 7. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1051] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 8. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 735] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 9. | GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 591] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 10. | Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, Hirao M, Yoshida K, Oki E, Sasako M. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the Matching package for R. J Stat Softw. 2011;42:1-52. [DOI] [Cited in This Article: ] [Cited by in Crossref: 750] [Cited by in F6Publishing: 738] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 12. | Sasako M, Inoue M, Lin JT, Khor C, Yang HK, Ohtsu A. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i28-i37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Society. Guidelines for Diagnosis and Treatment of Carcinoma of the Stomach. Available from: http://www.jgca.jp/pdf/Guidelines2004_eng.pdf. [Cited in This Article: ] |
| 14. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 399] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 15. | Van Cutsem E, Dicato M, Geva R, Arber N, Bang Y, Benson A, Cervantes A, Diaz-Rubio E, Ducreux M, Glynne-Jones R. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 2011;22 Suppl 5:v1-v9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Chang SH, Kim SN, Choi HJ, Park M, Kim RB, Go SI, Lee WS. Adjuvant Chemotherapy for Advanced Gastric Cancer in Elderly and Non-elderly Patients: Meta-Analysis of Randomized Controlled Trials. Cancer Res Treat. 2017;49:263-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1231] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 18. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 382] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 19. | Wang G, Zhao J, Song Y, Zhang W, Sun Y, Zhou A, Huang J, Du F, Yang L. Phase II study of adjuvant chemotherapy with S1 plus oxaliplatin for Chinese patients with gastric cancer. BMC Cancer. 2018;18:547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Comets E, Ikeda K, Hoff P, Fumoleau P, Wanders J, Tanigawara Y. Comparison of the pharmacokinetics of S-1, an oral anticancer agent, in Western and Japanese patients. J Pharmacokinet Pharmacodyn. 2003;30:257-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hu X, Chen L, Du Y, Fan B, Bu Z, Wang X, Ye Y, Zhang Z, Xiao G, Li F. Postoperative chemotherapy with S-1 plus oxaliplatin versus S-1 alone in locally advanced gastric cancer (RESCUE-GC study): a protocol for a phase III randomized controlled trial. Chin J Cancer Res. 2017;29:144-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Shen L. Phase III Study to Compare Perioperative Chemotherapy of Oxaliplatin Combined With S-1(SOX) Versus SOX or Oxaliplatin With Capecitabine (XELOX) as Post-operative Chemotherapy in Locally Advanced Gastric Adenocarcinoma With D2 Dissection. [accessed 2012 Feb 16]. In: ClinicalTrials.gov [Internet]. Beijing: Peking University. Available from: http://clinicaltrials.gov/ct2/show/NCT01534546 ClinicalTrials.gov Identifier: NCT01534546. [Cited in This Article: ] |
| 23. | Lin Y. SOX as Adjuvant Chemotherapy for Resectable Gastric Cancer. [accessed 2012 Mar 2]. In: ClinicalTrials.gov [Internet]. Beijing: Chinese Academy of Medical Sciences. Available from: http://clinicaltrials.gov/ct2/show/ NCT01542294 ClinicalTrials.gov Identifier: NCT01542294. [Cited in This Article: ] |
| 24. | Shen L. A Phaseâ ¡ Study: SOX vs SP in Adjuvant Chemotherapy After D2 Surgery. [accessed 2012 Sep 6]. In: ClinicalTrials.gov [Internet]. Beijing: Peking University. Available from: http://clinicaltrials.gov/ct2/show/ NCT01679340 ClinicalTrials.gov Identifier: NCT01679340. [Cited in This Article: ] |