Published online Aug 16, 2014. doi: 10.12998/wjcc.v2.i8.398
Revised: February 8, 2014
Accepted: June 13, 2014
Published online: August 16, 2014
As patients with carcinoma of the esophagus live longer, complications associated with the use of a gastric conduit are increasing. Ulcers form in the gastric conduit in 6.6% to 19.4% of patients. There are a few reports of perforation of a gastric conduit in the English literature. Almost all of these were associated with serious complications. We report a patient who developed a tension pneumothorax consequent to spontaneous perforation of an ulcer in the gastric conduit 7 years after the index surgery in a patient with carcinoma of the gastroesophageal junction. He responded well to conservative management. Complications related to a gastric conduit can be because of multiple factors. Periodic endoscopic surveillance of gastric conduits should be considered as these are at a higher risk of ulcer formation than a normal stomach. Long term treatment with proton pump inhibitors may decrease complications. There are no guidelines for the treatment of a perforated gastric conduit ulcer and the management should be individualized.
Core tip: We report a patient with a spontaneous perforation of an ulcer in the gastric conduit of a patient who had surgery for carcinoma of the gastroesophageal junction. He responded to conservative management with continuous decompression of the conduit with Ryle’s tube aspiration, proton pump inhibitors and enteral nutrition through a feeding jejunostomy for 4 wk. Periodic endoscopic surveillance should be considered as gastric conduits are at a higher risk of ulcer formation than a normal stomach and management of a perforated gastric conduit ulcer should be individualized.
- Citation: Patil N, Kaushal A, Jain A, Saluja SS, Mishra PK. Gastric conduit perforation. World J Clin Cases 2014; 2(8): 398-401
- URL: https://www.wjgnet.com/2307-8960/full/v2/i8/398.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i8.398
The stomach is preferred as the conduit after esophageal resection. Complications following gastric conduits are being reported more often as patients with carcinoma of the esophagus are living longer after resection. The incidence of an ulcer occurring in a gastric conduit is reported to be between 6.6% and 19.4%[1,2]. Perforation of a gastric conduit ulcer, although rare, may be catastrophic. The ulceration in a gastric conduit is often due to tumor recurrence. However, it may be due to other causes too. We report a patient with spontaneous perforation of a gastric conduit ulcer into the right pleural cavity that was successfully managed conservatively.
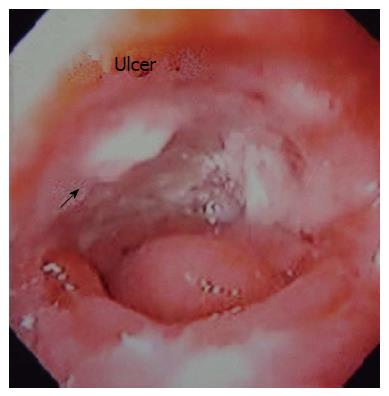
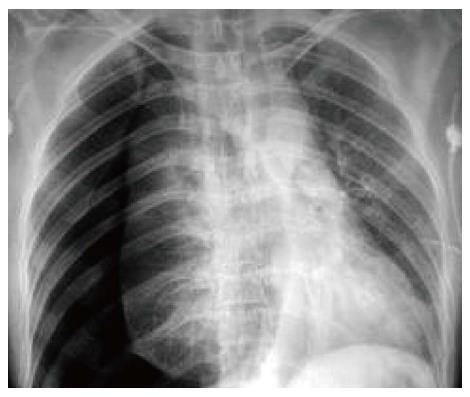
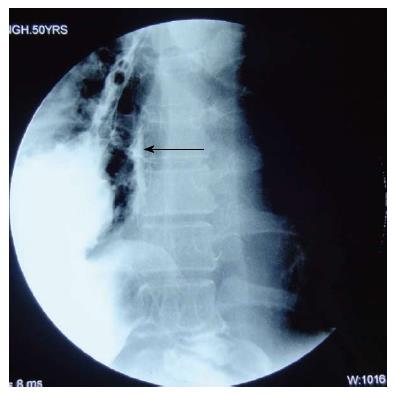
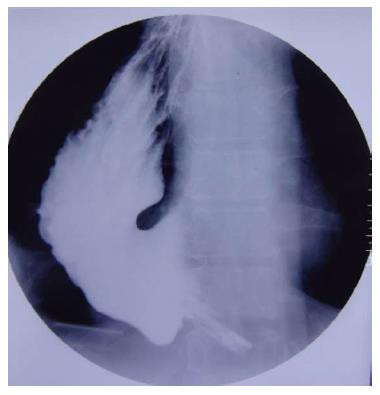
A 50-year-old man underwent a transhiatal esophagectomy and stapled cervical esophagogastric anastomosis without pyloromyotomy for carcinoma of the gastroesophageal junction in 2005. He had a minor anastomotic leak in the immediate postoperative period which was managed conservatively. The histology revealed a well differentiated adenocarcinoma of the gastroesophageal junction, infiltrating the adventitia. The resected margins were free of tumor and metastasis was seen in one of six lymph nodes. He did not receive any adjuvant treatment. In January 2006 he presented with dysphagia. A barium swallow revealed a stricture at the anastomotic site and an endoscopic biopsy did not show any local recurrence. The stricture was dilated with Savary-Gilliard dilators (Wilson Cook) up to 14 mm in two sessions and the patient became euphagic. He remained asymptomatic until June 2012 when he started complaining of pain in the neck and epigastric region. Endoscopy showed a large ulcer in the gastric conduit just below the anastomotic site. A biopsy from the ulcer did not reveal any malignancy (Figure 1). He was started on proton pump inhibitors (PPI) and Helicobacter pylori (H. pylori) eradication therapy. In July 2012, he had sudden onset of difficulty breathing and pain in the right side of the chest. At the time of presentation to our hospital the patient was hemodynamically stable. His hemoglobin was 13 g/dL, total leukocyte count of 16000 per cumm, and the blood urea and serum creatinine was 45 mg/dL and 1.2 mg/dL, respectively. The chest X-ray showed a tension pneumothorax on the right side with mediastinal shift to the left (Figure 2). The week before the patient had taken non-steroidal anti-inflammatory drugs for pain. A liter of purulent fluid with gastric contents was drained from the right hemithorax after insertion of an intercostal drainage (ICD) tube and his respiratory distress subsided. An oral Gastrografin study revealed a leak from the proximal part of the gastric conduit into the right hemithorax (Figure 3). A feeding jejunostomy was done because of the poor nutritional status of the patient. He was managed conservatively with continuous decompression of the gastric conduit using a Ryle’s tube (Romsins), antibiotics, PPIs, enteral nutrition through the feeding jejunostomy, serial chest X-rays and monitoring the ICD output. A follow up oral Gastrografin study at 4 wk revealed no evidence of any contrast leak from the gastric conduit (Figure 4). He was then allowed oral nutrition which he tolerated. There was no change in the nature and amount of the ICD fluid output. The ICD tube was subsequently removed and chest X-ray did not show any pleural effusion or pneumothorax. He is doing well with no symptoms at the 6 mo follow up. We did not manage this patient with insertion of an endoscopic stent as the leak was from the proximal part of the gastric conduit and the stent would have impinged on the cricopharynx. Stent migration was also likely because of the large diameter of the gastric conduit.
Increasing use of the stomach as a conduit has led to increasing reports of peptic ulcers in the conduit. In a prospective study of annual endoscopic evaluations in 114 patients who underwent gastric tube reconstruction after esophagectomy, 47% of patients had secondary gastric tube diseases, including gastritis [35.1% (40/114)], benign gastric tumors [10.5% (12/114)], gastric ulcers [6.1% (7/114)] and gastric adenocarcinoma [3.5% (4/114)][1]. Gastric tubes are reported to be at a higher risk of developing an ulcer than the normal stomach. The cause of a gastric conduit ulcer remains controversial. Several mechanisms have been postulated for the formation of gastric conduit ulcers, including normalization of the intraluminal pH profile over time, H. pylori infection (especially in patients with a history of peptic ulcer before surgery), delayed gastric emptying as a result of vagal denervation, bile reflux, ischemia due to mobilization of the gastric conduit, radiation, use of non-absorbable sutures and intake of non-steroidal anti-inflammatory drugs (NSAIDs), aspirin or steroids[3]. Most ulcers develop within 20 cm of the esophagogastric anastomosis, as in our patient, because the microcirculation is most disturbed in the upper part of the conduit[2]. The time for development of these ulcers has varied widely, from one month to as long as 150 mo.
Peptic ulcer of the gastric conduit can present with anemia, retrosternal or epigastric pain, fullness after eating or dysphagia[3]. It could be asymptomatic and vagotomy may be one of the reasons for the absence of pain[4]. A gastric conduit ulcer often causes serious complications, such as bleeding and perforation[5]. It may penetrate into any adjacent organ (left ventricular or atrial wall, thoracic aorta and other major vessels) or cavity, including the right pleural cavity, bronchi and pericardial cavity[5].
Only a few cases of gastric conduit perforation have been reported in the English literature and almost all of them had serious complications. More than half the patients were treated conservatively and all of them died[5]. All patients whose conduit ulcer perforated into the tracheobronchial tree or cardiovascular system died. Only patients with perforation into the sternum and thoracic cavity survived. Patients who had a gastric conduit perforation in the thoracic cavity underwent either primary closure of the perforated ulcer or resection of the ulcer followed by an interrupted closure buttressed with a pleural patch. Both these procedures are associated with high leak rates and mortality. In our case, the patient responded to conservative treatment, although we cannot recommend this for all cases.
Endoscopic surveillance should be done at least once every 6 mo as gastric conduits are at a higher risk of ulcer formation than a normal stomach and many such ulcers tend to be asymptomatic. Successful healing of a gastric ulcer by PPIs has been reported[1]. This could prevent potentially lethal complications associated with it.
While complications in the gastric conduit are being reported increasingly, there are no guidelines for the treatment of a perforated gastric conduit ulcer. These patients are usually sick and may not tolerate major surgery. The conservative management protocol cited above resulted in a good outcome in our case, showing that surgery is not always required and the management should be individualized. Avoidance of analgesics and periodic surveillance of the conduit may prevent complications.
The patient presented with sudden onset chest pain and difficulty breathing.
On clinical examination, decreased breath sounds in the right hemithorax with hyper resonant note on percussion.
Differential diagnoses were pneumothorax secondary to spontaneous rupture of pulmonary bullae, acute myocardial infarction and recurrence of disease.
Laboratory investigations were inconclusive.
On imaging, chest X-ray revealed right sided tension pneumothorax with mediastinal shift to left, gastric contents on insertion of intercostal drainage tube and oral Gastrografin study showed leak from the gastric conduit.
Previous endoscopy showed a large ulcer in the proximal part of gastric conduit, biopsy was consistent with peptic ulcer and also ruled out any recurrence.
He was treated conservatively with continuous decompression of the conduit through Ryle’s tube aspiration, proton pump inhibitors and enteral nutrition through feeding jejunostomy for 4 wk.
The possibility that ulceration in the gastric conduit may be due to causes other than tumor recurrence deserves greater recognition. Periodic endoscopic surveillance should be considered as gastric conduits are at a higher risk of ulcer formation than a normal stomach.
This is a rare morbid complication of gastric conduit which responded to conservative management. However, a firm conclusion cannot be drawn on the management guidelines of perforated gastric conduit ulcer and treatment should be individualized.
P- Reviewer: Abd Ellatif ME, Boyacioglu AS, Gonzalez AM, Marangoni G S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, Imano H, Inoue Y, Ogawa J. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepatogastroenterology. 2003;50:666-669. [PubMed] [Cited in This Article: ] |
| 2. | Suzuki H, Saito R, Sasaki S, Okuyama M. Analysis of the cases with peptic ulcer of gastric tube after esophageal replacement for esophageal cancer. Rinsho. 1999;54:1075-1079. [Cited in This Article: ] |
| 3. | Piessen G, Lamblin A, Triboulet JP, Mariette C. Peptic ulcer of the gastric tube after esophagectomy for cancer: clinical implications. Dis Esophagus. 2007;20:542-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Texter EC. Ulcer pain mechanisms. The clinical features of active peptic ulcer disease and implications for therapy. Scand J Gastroenterol Suppl. 1987;134:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ubukata H, Nakachi T, Tabuchi T, Nagata H, Takemura A, Shimazaki J, Konishi S, Tabuchi T. Gastric tube perforation after esophagectomy for esophageal cancer. Surg Today. 2011;41:612-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |