Published online Jun 16, 2014. doi: 10.12998/wjcc.v2.i6.172
Revised: April 21, 2014
Accepted: May 16, 2014
Published online: June 16, 2014
Processing time: 110 Days and 10.1 Hours
Human papillomavirus (HPV) has been implicated in the pathogenesis of a subset of oropharyngeal squamous cell carcinoma. As a result, traditional paradigms in relation to the management of head and neck squamous cell carcinoma have been changing. Research into HPV-related oropharyngeal squamous cell carcinoma is rapidly expanding, however many molecular pathological and clinical aspects of the role of HPV remain uncertain and are the subject of ongoing investigation. A detailed search of the literature pertaining to HPV-related oropharyngeal squamous cell carcinoma was performed and information on the topic was gathered. In this article, we present an extensive review of the current literature on the role of HPV in oropharyngeal squamous cell carcinoma, particularly in relation to epidemiology, risk factors, carcinogenesis, biomarkers and clinical implications. HPV has been established as a causative agent in oropharyngeal squamous cell carcinoma and biologically active HPV can act as a prognosticator with better overall survival than HPV-negative tumours. A distinct group of younger patients with limited tobacco and alcohol exposure have emerged as characteristic of this HPV-related subset of squamous cell carcinoma of the head and neck. However, the exact molecular mechanisms of carcinogenesis are not completely understood and further studies are needed to assist development of optimal prevention and treatment modalities.
Core tip:Human papillomavirus has been accepted as a causative agent in a subset of head and neck squamous cell carcinoma (SCC), particularly of the tonsils and base of tongue. Importantly, there is an increasing incidence of this subset of patients, who demonstrate improved prognosis and may respond more favourably to treatment. Similarities and differences are evident between cervical and oropharyngeal human papillomavirus-related SCCs and the comparison between these tumours warrants further investigation to better understand the disease process.
- Citation: Woods RS, O’Regan EM, Kennedy S, Martin C, O’Leary JJ, Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World J Clin Cases 2014; 2(6): 172-193
- URL: https://www.wjgnet.com/2307-8960/full/v2/i6/172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i6.172
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common type of cancer worldwide with approximately 633000 new cases diagnosed and 355000 deaths annually[1]. Over the past 10-15 years, the traditional paradigms of HNSCC have been changing significantly. It has emerged as a heterogeneous group of diseases, with distinct molecular genetic changes[2,3].
Human papillomavirus (HPV) has been linked to the pathogenesis of squamous cell carcinoma (SCC) since the 1970s[4] and, in 1995, it was recognised by the International Agency for Research on Cancer (IARC) that high risk HPV types 16 and 18 were carcinogenic in humans[5]. The role of HPV in cervical cancer is well described[6], however high risk HPV types are also linked with other ano-genital tumours and with SCCs of the head and neck[7,8], as well as potentially playing a role in cutaneous SCCs[9]. HPV accounts for roughly 4.8%-5.2% of the total global cancer burden, making it the highest among all viruses[10,11].
Since it was first suggested in 1983[12] and first identified in 1985[13], HPV infection has been increasingly recognized as a major aetiologic factor for HNSCCs, particularly a subset that arise from the oropharynx, mostly the base of tongue and palatine tonsils[14-16]. This subset is seen as a distinct clinicopathological entity in comparison to the traditional smoking and alcohol related HNSCCs[16-20]. Specific genetic changes induced through HPV E6 and E7 protein expression define this subset[21-23]. In contrast, tobacco associated HNSCCs are usually more genetically diverse[24]. HPV-related tumours of the oropharynx display specificity of HPV to the tumour cell nuclei[16], integration of HPV DNA into the host cell[16,25] and high viral copy numbers[26], giving evidence for the functional role of HPV in the pathogenesis of these tumours.
HPV-related SCC tends to display unique histology characterized by poorly differentiated, non-keratinising morphology with a basaloid appearance[17,27]. Nevertheless, even some true basaloid squamous cell carcinomas of the oropharynx have demonstrated HPV-positivity[28], and other variants such as papillary SCC, adenosquamous carcinoma, lymphoepithelial carcinoma-like tumours and small cell carcinoma have been associated with HPV infection[29-34].
It is estimated that the probability of a cancer of the oropharynx being attributable to HPV is five times higher than the oral cavity, larynx or hypopharynx[35], with HPV-related oropharyngeal SCC being described as an epidemic[36-40]. Current data from studies that assessed in situ hybridization or HPV E6/E7 mRNA suggest that HPV-related HNSCC is rare in the oral cavity, larynx, hypopharynx and other HNSCC sites[35], however the role of HPV in non-oropharyngeal sites remains unclear[41] and a causative relationship at these sites has not been established[42].
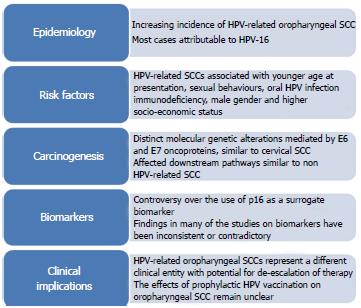
We review the current literature regarding HPV-related oropharyngeal tumours with regard to epidemiology, risk factors, carcinogenesis, biomarkers and clinical implications. A summary is shown in Figure 1.
HPV is an epitheliotropic, non-enveloped DNA virus measuring approximately 55 nm in diameter, and carries a single molecule of circular double-stranded DNA, consisting of approximately 8000 base pairs[43]. The genome is broken down into three regions which consist of a long control region (LCR), an early (E) region and a late (L) region. There are eight genes in the E region and two in the L region. These genes in E and L encode viral proteins while LCR is an upstream non-coding regulatory region containing the origin of viral DNA replication and transcriptional regulatory elements.
At present, over 200 different genotypes of papillomaviridiae, characterized by at least 10% nucleotide divergence in capsid gene (L1)[44], have been identified by various techniques[45]. These can be classified according to similarities in their DNA sequences. They have also been grouped into mucosal (mostly of the alpha genus) or cutaneous (mostly of the beta genus) types based on their tropism for specific epithelia and they can be classified into low and high risk types based on their capacity to promote malignant transformation in host cells. Of these, HPV 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73 and 82 are examples of those classified as high risk viruses, detectable in high grade squamous intraepithelial lesions in the cervix or in invasive cancer; while HPV 6, 11, 40, 42, 43, 44, 54, 61, 72, 81, and 89 can be considered as viruses with low oncogenic risk and can be isolated from low grade epithelial lesions of the cervix. There remain a number of HPV types that are potentially high risk with an unknown oncogenic potential. There exists some degree of intratypic variation[46,47], which may also relate to pathogenesis[48-50], as well as geographic variation in genotype prevalence[46,51].
HPV is one of the most powerful human carcinogens. The E6 and E7 genes produce E6 and E7 oncoproteins, which confer the virus with oncogenic potential through their inhibitory effects on p53 and retinoblastoma (Rb) proteins, more of which is discussed later.
HNSCC includes tumours from a number of subsites, of which the oropharynx accounts for approximately 10%[52]. Worldwide, there were an estimated 85000 new cases of oropharyngeal SCC in 2008, of which 25.6% (22000) were estimated to be HPV-related[53]. Of the HPV-related cases, more than three quarters (17000) were estimated to be male.
Genotypes of oncogenic HPV found in cervical cancer in order of prevalence are 16, 18, 58, 33, 45, 31, 52, 35, 59, 39, 51 and 56[54]. However, the distribution of HPV types differs somewhat in oropharyngeal when compared to cervical cancers[55]. A systematic review found that HPV-16 was present in 95.7% of HPV-related oropharyngeal SCC, but only 73.9% of HPV-positive non-oropharyngeal HNSCCs[56], while only approximately 61% of cervical cancer display HPV-16[57]. While a significant number of other oncogenic HPV types are found in cervical cancer, only a small proportion of oropharyngeal cancers may be caused by additional HPV types such as 18, 31, 33, 35, 52 and 58[58,59]. HPV-16 is the commonest genotype found in oral cavity infection[60], while it constitutes over 90% of the genotype distribution in tonsil cancers[61].
Prevalence of oral high risk HPV infection in the general population is reported at 3.5%-3.7%[62,63], with higher rates for those also infected with HIV[64]. A systematic review of the literature in 2005 reported detection of HPV DNA in 35.6% of oropharyngeal tumours[65]. However, there exists a wide geographic variation, with a reported prevalence as high as 72%[59] in North America compared to 17% in southern Europe[53], 12.6% in Taiwan[66] and even as low as 4.4% reported in central Europe and Latin America[67]. Some of these figures are based on the assumption that detection of high-risk HPV DNA in tumour tissue signifies cancer attributable to HPV, however this does not delineate from the effects of tobacco exposure and alcohol in these cases. It has been recorded that HPV accounts for approximately 7.7% and 2.2% of all cancer cases in developing and developed countries, respectively[10]. The variations could partly be explained by geographic and temporal heterogeneity in sexual behaviours and tobacco exposure[41]. A more recent systematic review in 2012 reported a prevalence of HPV in oropharyngeal SCC of 59.9% in the United States, compared to 39.7% in Europe and 32.5% in the rest of the world[56]. There are limited data from less developed regions, but the incidence appears much lower.
Despite the variation in prevalence, case control studies conducted around the world show strong and consistent associations of markers of HPV exposure with risk of oropharyngeal cancers, even after adjustment for important HNSCC risk factors such as age, gender and tobacco and alcohol use[41].
While incidence of other HNSCCs has decreased over the past two decades, correlating with decreased tobacco use, the age-adjusted incidence of oropharyngeal SCC has been increasing in this same period[68,69], particularly of the base of tongue and tonsil region[70]. Meanwhile the population-level incidence of HPV positive oropharyngeal SCC increased by 225% between 1988 and 2004, with a concomitant decline by 50% for HPV-negative oropharyngeal SCC[59]. A particularly steep rise of over 70% has been reported for prevalence of HPV-related oropharyngeal SCC in the past decade, with prevalence in Europe increasing at a faster rate than North America[56]. This rise further emphasises the predilection of HPV for the oropharynx and suggests that it plays a less significant role in other HNSCCs.
With the rise in HPV-related oropharyngeal SCC coupled with the decline of HPV-related cervical SCC, it has been suggested that the annual numbers of HPV-related oropharyngeal cases could soon surpass that of cervical cancer[41].
HNSCCs, including those of the oropharynx, have traditionally been strongly associated with patients who have a long history of heavy smoking and alcohol consumption, with previous studies clearly showing a dose-response relationship with the frequency and duration of tobacco and alcohol exposure[71]. Age of onset is generally in an older age group (usually seventh decade) in these traditional HPV-negative oropharyngeal SCCs. Other risk factor associations with these tumours include poor oral hygiene[72,73], a diet low in fruit and vegetable consumption[74,75] and chronic inflammatory disease in the oral cavity[76-78].
The distinct subset of HPV-positive oropharyngeal SCCs generally present at a younger age, averaging a few years lower than HPV-negative tumours[39]. Although phenotypically similar to those in older patients, HNSSCs developing in younger patients are undoubtedly different at a genetic level with both germline and somatic differences seen[3,79-82]. One study showed that patients under 55 had a 3.4-fold higher risk of infection with carcinogenic HPV[83], while a strong association has been demonstrated with HPV-16 infection and tonsillar cancer in males under 40 years old[84]. Increasing incidence of oropharyngeal SCC is seen in those aged under 60[85], with a particularly steep rise seen between the ages of 50-59[86], although it is possible this may be due to other risk factor exposures in this birth cohort.
HPV-related oropharyngeal SCCs also show strong associations with sexual behaviours, correlating with disease[87]. In a large number of studies, both HPV-positive HNSCCs and oropharyngeal SCCs have been strongly associated in comparison to other HNSCCs with number of lifetime sexual partners, number of vaginal, oral and anal sexual partners, young age at first intercourse/earlier sexual contact and history of sexually transmitted diseases, including genital warts[27,83,87-93]. After adjusting for HPV-16 serology, the associations in a case-control series were no longer significant, suggesting that sexual behaviours can be seen as a surrogate for HPV-16 exposure[27].
Data from a number of developed countries show that markers of high-risk sexual behaviours, such as earlier ages of sexual debut, practice of premarital sex, average number of lifetime partners, and practice of oral sex, have all increased among recent birth cohorts[94].
Oral HPV is predominantly acquired via sexual transmission and oral HPV prevalence has been associated with some of the above sexual behaviours. Studies have demonstrated increased HPV acquisition around sexual debut with oral HPV prevalence of 1.5% in 12-15 year olds, 3.3% in 16-20 year olds and 4.5%-6.9% in healthy adults[62,63,89,95]. Higher oral HPV prevalence has been reported in women with cervical HPV infection[96,97], and people infected with Human Immunodeficiency Virus (HIV)[96,98]. Several studies and some case reports have described concordant oral HPV infection between couples[99-102], however preliminary results from the HPV oral transmission study in partners over time (HOTSPOT) have not backed up these findings.
It has even been suggested that non-sexual HPV transmission through kissing may be possible[95,103], as well as intrapartum transmission[104] and transmission during laser surgery[105]. In itself, oral HPV-16 infection is a strong risk factor for oropharyngeal cancer, while the relationship is not necessarily clear for oral SCCs[106,107]. However, oral HPV prevalence is lower than cervical, perhaps explained by a lower proportion in oral-genital than genital-genital partners[55], but the natural history of HPV infection in the oral cavity appears similar to cervical infections[108]. Although type-specific concordance is low, HPV infection of the cervix and oral cavity are not independent[109] and so cervical HPV infection could be considered a risk factor for oral cavity HPV infection. Although the full natural history of HPV infection in the oral cavity and oropharynx is not entirely understood, there is an estimated incidence of 4.4% per year with most infections being cleared within one year[110]. However, changing sexual practices are potentially leading to higher rates of infection that could become recalcitrant to immune responses.
Evidence of a role for tobacco exposure and alcohol use in HPV-related oropharyngeal SCCs and in oral HPV infection is equivocal, with some studies reporting positive association and suggesting smoking-induced immunosuppression or potentiation of carcinogenesis could play a role, while others report no association[41]. A role for tobacco smoking in cervical cancer, however, has been demonstrated, although this association becomes weak after adjustment for sexual and reproductive factors[111]. In comparison to traditional HNSCCs, these patients are less likely to have excessive tobacco exposure and alcohol use[16,88,112], however HPV-related oropharyngeal SCCs do occur in both in those with tobacco exposure and alcohol use and in those without. It is highly plausible that tobacco exposure potentiates the effects of HPV carcinogenesis[113] but a role in the causation of HPV-related oropharyngeal SCCs has not been definitively determined from available evidence[35]. Marijuana use has also been associated with oropharyngeal SCCs[87,114], however after adjustment for sexual behaviour variables in one study, this disappeared[62].
Both HPV-related and non HPV-related HNSCC exhibit male predominance at a ratio of approximately 3:1. In tobacco and alcohol related HNSCC, this difference has decreased particularly as trends in smoking have changed, with 43% of men and 30% of women smoking in 1974 compared to 26% of men and 21% of women in 2000[115]. Nonetheless, the difference still remains for HPV-related HNSCC and the reason for this is uncertain. The male predominance exhibited cannot be fully explained by difference in sexual behaviours, which suggests potential biologic differences between men and women[41,116], or that some male characteristic preferentially predisposes to cancer of the oropharynx[117]. It has been suggested that hormonal differences[55,118] or the potential protective immunity from seroconversion in response to cervical HPV infections among women[119,120] may play a role. Although not all studies agree[63], the majority of studies report that oral HPV infection is more common in men than women[62,121,122]. It has also been suggested that transmissibility of oral HPV may be higher for men performing oral sex on women, possibly due to a higher HPV copy number in the vagina/cervix[94].
Immunodeficiency is a risk factor for a large number of tumours and HPV-related oropharyngeal SCC is included in that. For example, it is reported that patients infected with human immunodeficiency virus (HIV) have a 2-6 times increased risk of HPV-related HNSCC[123,124], although they are at greater risk of ano-genital SCCs than oropharyngeal[125]. It has been demonstrated in cervical cancer patients that immunosuppression leads to HPV persistence and disease progression[126-128]. The association of a deficient immune system with increased HPV-related HNSCC may partly explain any potential association with tobacco exposure due to the immunosuppressive effects of smoking[129], with one paper demonstrating a reduced antibody response in smokers[130].
HNSCCs have been associated with patients from a low socio-economic group for many years[131]. However, HPV-related oropharyngeal SCCs are associated with patients who are from a higher socio-economic group and who have a better performance status[132,133], although this has been refuted in one study[116]. Nonetheless, white males seem to be particularly at risk, with a rise in incidence reported in this group alone[59,85,116]. HPV positivity in oropharyngeal cancer is lower in African Americans than in other racial groups, with poorer survival in this racial group from oropharyngeal SCC, because a higher proportion is related to tobacco and alcohol exposure[134,135].
There is a strong association between serologic evidence of HPV infection and HNSCC risk, even after adjustment for other HNSCC risk factors[106]. One study has even shown a temporal association, with pre-diagnostic serum samples from ten years prior that were positive for HPV-16 capsid antibodies conferring an increased risk of oropharyngeal SCC of 14.4[136], while patients with pre-diagnostic E6 seropositivity had a significantly higher risk of oropharyngeal cancer in another study[137].
It is evident that a number of factors can facilitate or increase the risk of HPV-related oropharyngeal SCCs. This includes oral HPV infection, male gender, younger age, white race, immunosuppression and a variety of sexual behaviours. Differences in sexual behaviours across age and gender and consequent HPV exposure risk could account for the rapidly increasing incidence of HPV-related oropharyngeal SCCs among younger patients. Interestingly a separate specific subgroup of younger females with non HPV-related oral cavity SCCs has also been identified[138].
The model for development of SCC involves exposure to carcinogens over time leading to progressive genetic and epigenetic changes that accumulate and lead to premalignant and eventually malignant lesions. However, HNSCC is a heterogeneous disease with a number of subtypes described, based on histological appearance, and supported by different gene expression profiles[139,140]. Squamous cell carcinomas from different sites in the body share a number of molecular characteristics but recent whole-exome sequencing[141-144] has helped to characterise the specific molecular pathogenesis of HNSCC with roles identified for tumour suppressor pathways including p53, Rb/INK4/ARF and NOTCH[145]. A role for cancer stem cells in HNSCC is likely, based on recent evidence[146-149], and further study of these progenitor cells will help to elucidate mechanisms of carcinogenesis.
Recent deep-sequencing studies on the HNSCC oncogenome have demonstrated a vast number of diverse genetic alterations, however most of these converge on four targetable molecular pathways[150]; mitogenic signalling and in particular amplification or up-regulation of epidermal growth factor receptor (EGFR) and the downstream pathway of phosphoinositide 3-kinase (PI3K)/mTOR as well as PTEN inactivation, each leading to pathways involving proliferation, DNA repair, survival and spread; defective differentiation involving NOTCH signalling alterations; cell cycle de-regulation involving inactivation of CDKN2A (encoding p16 INK4A ) tumour suppressor gene and CCND1 (encoding CYCLIN D1) amplification; genomic instability involving loss of TP53, which occurs in a large percentage of non HPV-related HNSCC and is the single most common mutational event, and other genes related to DNA damage recognition and repair. It is possible that smoking and alcohol affect distinct genes[151], giving further evidence for a synergistic effect of tobacco and alcohol exposure in relation to HNSCC carcinogenesis.
HNSCC usually displays field cancerisation, a term first coined in 1953[152], whereby specific genetic alterations can be widely distributed throughout the mucosa lining the aerodigestive tract even in the absence of overt histopathologic changes of malignancy[25]. Only a minority of precancerous fields in the oral cavity are recognised as leukoplakia or erythroplakia[153] and only 6%-36% of patients with leukoplakia or erythroplakia go on to develop oral SCC[154], particularly those demonstrating aneuploidy[155,156]. The accumulation of further genetic changes in precancerous fields leads to the development of SCC, with presence of field change leading to a higher risk of multiple synchronous or metachronous primary tumours. Exposure to carcinogens bring about these field changes, however evidence for a field effect is lacking for HPV-related SCC[157] and the risk of second primary malignancy in oropharyngeal SCC has markedly decreased over time[158], with the mutation rate of HPV-positive tumours only approximately half of that found in HPV-negative HNSCC[141,142].
Specific differences in chromosomal alteration and gene transcription have been identified between HPV and non HPV-related HNSCCs[21,22,159,160]. TP53 mutations, loss of 9p21, hypermethylation of 14-3-3σ and RASSF1A promoters and overexpression of cyclin D are all common in non HPV-related oropharyngeal SCCs, while pRb levels are normal and p16 is often decreased[161,162].
In the cervix, after initial infection at the transformation zone, viral genomes are maintained as episomes in the basal layer, with viral gene expression being tightly controlled as the infected cells move toward the epithelial surface[163]. Subsequent high-grade neoplasia represents an abortive infection in which viral gene expression becomes deregulated and the normal life cycle of the virus cannot be completed. The squamous epithelium in the cervix and the head and neck derive embryologically from endoderm and are susceptible to metaplasia[164]. In the head and neck, there is a predilection for HPV-positive tumours to occur in the reticular crypt epithelium of palatine and lingual tonsils and head and neck sites with mucosa associated lymphoid tissue[25,165,166]. It is possible that this occurs due to the particular microanatomy of the crypts, where there are breaks in the non-keratinising squamous epithelium that could allow viral entry, while a microabrasion theory of entry to basal cells at other head and neck sites has been proposed. Entry may be facilitated by M-cells lining the crypt epithelium[167], as with other viruses[168,169]. Another theory postulated is an influence on HPV carcinogenesis from increased cytokines related to nearby lymphoid tissue[170]. The recent observation of a distinct set of embryonic cells at the squamocolumnar junction of the cervix, which seem to confer a particularly high risk of malignancy, has led to a “top-down” theory of malignancy at this site, although it remains to be seen if this model translates to the oropharynx[171-173]. Despite being full of lymphatic tissue, the tonsils are known to harbour pathogenic viruses such as Epstein Barr virus, adenoviruses and herpes simplex virus[174], and it is the mechanisms of immune evasion that allow persistent infection and carcinogenic potential at these sites, hence immunosuppressed individuals are particularly at risk.
From cervical models, we understand that most HPV infections last no more than a few months and are eliminated by the immune response, with 90% of infections cleared within two years, although high risk HPV tends to persist longer than low risk[175,176]. Once immune evasion is established, integration of HPV DNA into the cellular genome likely represents a critical step for malignant transformation in those individuals who harbour HPV in their tonsils[25], with HPV integration representing a stochastic process resulting in clonal selection of aggressively expanding cells that display altered gene expression of integrated HPV genomes and potential perturbations of cellular genes at or near viral integration sites[177]. Viral integration can also lead to loss of E2-mediated inhibition of viral oncoprotein expression[178]. Furthermore, it has been shown that this HPV DNA integration is consistently centred on tonsil crypt epithelium[25], however the factors allowing transformation from episomal HPV infection, whether active or latent, to DNA integration remain poorly understood. It has also been noted that much of the HPV that is detected in oropharyngeal cancers seems to be episomal.
Based on cervical cancer models, high-risk HPV can induce genetic changes in a small number of those with persistent infection which leads to precancerous lesions, a fraction of whom will develop cancer many years after the original infection. While HPV-related precursor lesions in the oral cavity have been identified[179], there is an absence of detectable precancerous lesions in the oropharynx[41], perhaps related to the difficulty in assessing and sampling deep tonsillar crypts, the predominant location of HPV-related SCCs[180,181]. Nevertheless, HPV-related oropharyngeal SCCs present with distinct molecular profiles, more comparable to cervical SCC than to non HPV-related HNSCC[55]. Infection with HPV is likely an early oncogenic event in HNSCCs. The viral oncoproteins E6 (151 amino acids) and E7 (98 amino acids) of high risk HPV types, particularly HPV-16, are implicated as the drivers of transformation in HPV-related oropharyngeal SCCs[182]. These proteins help to re-program postmitotic terminally differentiated epithelial cells to re-enter the cell cycle and express proteins that are required for viral genome replication[183]. They also disrupt a number of cellular mechanisms through a wide variety of downstream effects.
The E5 oncoprotein co-operates with E6 and E7 to promote proliferation of infected cells and is likely to facilitate malignant progression[184], although this process is likely to take place in the early stages of carcinogenesis because viral integration frequently leads to loss of E5 gene expression[185]. Transcription of E6 and E7 viral oncogenes can occur when the virus is episomal however, in cervical SCC, alteration of E2 on integration may facilitate increased expression of E6 and E7 oncogenes, although this may not be the case in oropharyngeal SCC[186]. Viral integration is thought to play an important role in cervical SCC but the relevance of viral integration is not fully clear in oropharyngeal SCC[187]. Some studies suggest that viral integration in the tonsillar crypts plays an important role in carcinogenesis[165,188], which may explain the predilection of HPV-related HNSCCs at this site, while other studies suggest that episomal HPV alone contributes to the development of most oropharyngeal SCCs in contrast to SCCs of the cervix[186,187].
In cervical lesions, it is not possible to predict tumour progression based on HPV viral load[189]. It has been suggested that high HPV viral load (at least one HPV copy per tumour cell) in oropharyngeal SCC predicts active HPV infection[190-192]. The proportion of HPV-positive SCCs with high viral load varies between studies from 33%-77.5%[59,190]. It is possible that in cases of low viral load that HPV presence is coincidental and alternative mechanisms of carcinogenesis are implicated. However, gene expression varies widely and so a constitutive rather than a high expression of viral oncogenes may be all that is required for HPV-related oropharyngeal carcinogenesis[187].
The major role of E6 oncoprotein is induction of ubiquitin-mediated proteolysis, through E6 associated protein, leading to degradation of tumour suppressor p53. As p53 usually facilitates repair to damaged host DNA by arresting cells in the G1 phase (or else inducing apoptosis), E6 expressing cells face increased mitotic stress and genomic instability[193]. E6 aids cellular proliferation by up-regulating transcription of telomerase[194] and also, through the presence of the PDZ binding motif, high risk HPV E6 proteins bind to a number of PDZ domain containing proteins with presumed tumour suppressor activity that have diverse functions[183,195]. E6 also targets the Wnt and Notch signalling pathways[183].
The E7 oncoprotein causes cell cycle disruption by binding and inactivating tumour suppressor proteins of the retinoblastoma family (pRb) that regulate cellular senescence. E7 thereby causes cell proliferation through abnormal entry into the S-phase by the overexpression of released transcription factor E2F[196]. This functional inactivation of pRB also results in overexpression of p16 tumour suppressor protein, which is a CDK4A inhibitor, allowing the use of p16 as a surrogate marker for HPV-related oncogenesis[39,197-200], which will be discussed further below. E7 proteins also alter cell cycle control through interactions with histone deacetylases, cyclins and cyclin-dependent kinase inhibitors[201].
Animal models suggest that E7 is the dominant HPV oncoprotein in HNSCC[202], but both E6 and E7 directly impact upon a number of apoptotic mechanisms; interaction with extracellular matrix adherence proteins to allow anchorage independent growth; interaction with cell surface receptors to resist cytokine induced extrinsic apoptosis; and interaction with proteins involved in interferon signalling and interleukin to allow immune evasion[201,203].
Genomic instability underpins the development of dysplasia, malignancy, invasion, and metastasis in cancers[204]. While aberrant proliferation induced by E7 is facilitated by suppression of apoptosis by E6 mechanisms, it is the additional functions of E6 and E7 to induce genomic instability by multiple mechanisms that lead to chromosomal mutations. These include centrosome abnormalities or spindle checkpoint failure leading to polyploidy, aneuploidy and chromosomal rearrangement[205,206], direct DNA damage[207] (which also occurs with viral integration[208]), variation in the Fanconi anaemia DNA repair pathway and induction of the ATM-ATR DNA damage repair pathway with concomitant disruption of checkpoint control mechanisms[201]. Tobacco exposure also causes genomic instability and so may help to induce malignancy on the background of E6 and E7 effects, allowing for a role of tobacco exposure in the potentiation of HPV-related HNSCC, which has been suggested from mouse models[209].
Different patterns of DNA methylation have been demonstrated between HPV and non HPV-related HNSCCs, with methylation patterns in HPV-related HNSCCs more analogous to cervical SCC patterns than non HPV-related HNSCCs[210]. Excess DNA methylation could be recruited by the integrated viral genome rendering it invisible to host immune responses or it could be an attempted defence mechanism by the host cell[210]. HPV-related HNSCCs also have a distinct miRNA profile, also more analogous to cervical SCCs, in comparison with non HPV-related HNSCCs[211]. Furthermore, differences in DNA methylation rate have been identified between HNSCCs in tobacco users versus nonusers as well as specific mRNA and microRNA clusters[212].
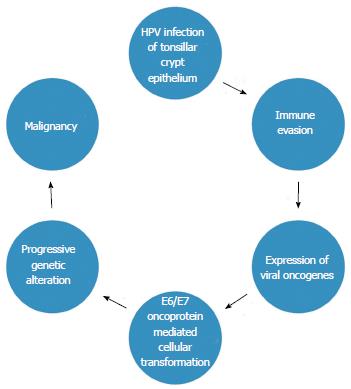
While distinct methods of carcinogenesis are evident between HPV-related and non HPV-related HNSCCs, the effects on downstream pathways are often the same, such as in the case of mTOR inhibition, either from TP53 mutations in tobacco related cases or from E6 induced degradation of p53 in HPV-related cases[150]. It is also important to note that E6 and E7 proteins expressed in low risk HPV types do not induce the same changes and that HPV present in some HNSCCs may exist as a latent passenger virus with no transcriptional activity[213,214]. New roles for HPV oncoproteins are continually being identified, offering many future potential therapeutic targets[215]. In any case, there is a distinct group of HPV-related tumours arising from the epithelium of lymphoid tissue characterised by viral oncoprotein expression, rather than SCCs that arise on a background of a long history of somatic mutations due to carcinogenic exposures. A proposed model of carcinogenesis in HPV-related oropharyngeal SCC is shown in Figure 2.
Studies have had difficulty identifying clinically useful biomarkers in HNSCC[216]. A high degree of heterogeneity is evident in HNSCC, with different prognosis described for different subsets of tumours. This includes a favourable prognosis for the growing cohort of HPV-related SCCs, particularly oropharyngeal[217], often despite a more advanced presentation. This is due to a number of factors including the sensitivity of this subset to chemoradiation[133], the lower likelihood of loco-regional recurrence[217] and a younger cohort of patients with fewer co-morbidities as well as a possible decreased risk of second primary tumours.
HPV status and p16 status have each proven useful as biomarkers in HNSCC. The tumour suppressor p16 binds to the cyclin D1 CDK4/CDK6 complex, thereby helping to keep the Rb protein in its active hypophosphorylated form. With pRb functionally inactivated by the binding of HPV E7 oncoprotein, p16 expression is upregulated by its corresponding gene being released from transcriptional inhibition. In non HPV-related HNSCC, downregulation or loss of p16 protein expression is a common early event and is associated with a worse prognosis, consistent with the tumour-suppressor role it has[204], and oral cavity and hypopharyngeal SCC show lower levels of p16 positivity[218,219]. However, a strong correlation has been observed in numerous studies between integrated HPV detection and p16 protein overexpression. As such, p16 has been adopted as a surrogate biomarker for HPV-related HNSCC[39,197-200,220,221], with immunohistochemistry for p16INK4A now routinely performed in many laboratories and guides for interpretation have been described[199].
Not only can p16 act as a surrogate biomarker for HPV status, with 46%-98% of HPV positive oropharyngeal SCCs demonstrating p16 positivity on pooled analysis[200], but, with 3%-51% of HNSCCs being p16 positive and HPV negative[200], p16 status can also act as an independent prognosticator, regardless of HPV status[222-225], although not all studies agree on the specific effect[214]. Overexpression of p16 has been found in normal tonsillar tissue[25,226] and HPV negative tumours, with dysregulation of epigenetic control or multiple transcription factors being other mechanisms that lead to aberrant expression of p16[227], some of which are associated with non HPV-related HNSCC carcinogenesis. The lack of clarity on p16 expression and discrepancies in interpretation of p16 IHC have led to controversy surrounding its use as a surrogate biomarker.
Overexpression of p16 is not evident in a subgroup of HNSCC with active HPV infection[228], 2%-54% in pooled analysis[200]. With overexpression of p16INK4A thought to represent activity of viral oncogenes, it is possible that HPV positive/p16 negative may represent latent HPV infection, which could explain why HPV positive/p16 negative HNSCCs have a slightly worse prognosis[229,230]. Therefore, by combining testing for HPV DNA positivity and p16 overexpression, one can eliminate cases related to inactive infection, improving specificity of p16 a surrogate biomarker for detection of biologically relevant HPV infection[200]. This has been shown to be as reliable as detection of HPV E6⁄E7 mRNA expression by polymerase chain reaction, which is considered the gold standard of testing for transcriptionally active virus because HPV-negative HNSCCs and HPV-positive⁄E6 and E7 mRNA-negative HNSCCs show similar survival curves[191]; however E6/E7 mRNA is only used in a small number of centres and is mostly restricted to the research laboratory[231]. Equivalent detection may also be possible with HPV mRNA ISH and this may be of more practical clinical use[232,233]. However, there is currently a great degree of heterogeneity of HPV assessment techniques used in clinical practice depending on location[234].
A large number of alternative prognostic or predictive biomarkers in HNSCC have been studied, such as EGFR, cyclin D1, Bcl-2, cyclin-dependent kinase inhibitor p27, MCM7, DSG3, vascular endothelial growth factor, p53, ERCC1, RRM1, β-catenin and MET[209,235-240]. Some examples of studies on biomarkers related to treatment response found MMP-7 and EGF to be predictive markers of, respectively, resistance to cisplatin and poor response to cetuximab[241-243], while survivin overexpression predicted improved response to radiotherapy[244]. However, findings in many of the studies on biomarkers have been either inconsistent or often even contradictory. Some biomarkers have not been studied in sufficient detail to draw a firm association[216]. However, EGFR positivity has been associated with poor survival in HNSCC in a number of studies[245-247], including in HPV-related HNSCCs[167,235] (although these tumours generally tend not to overexpress EGFR[248]), and an EGFR-targeting antibody, cetuximab, has shown benefit in combination with radiotherapy for patients with HNSCC[249] so is approved for clinical use alone or in combination with radiotherapy or chemotherapy. Evidence suggests p16 may be useful in the context of analysing treatment response to cetuximab[250]. Besides EGFR inhibition, new molecular targeted therapies that have an effect on other activated molecular signalling pathways such as mTOR, Src kinase and IGF-1R inhibitors are being developed[251].
In HPV-related oropharyngeal SCC, there is the potential for translation of cervical biomarkers given the similarities in carcinogenesis between these sites. New biomarkers are continually emerging from molecular biological research which are of as yet uncertain relevance, such as recently discovered distinct squamocolumnar junction-related biomarkers[171-173].
It has been suggested that the programmed death 1 (PD-1):PD-L1 pathway plays a role in HNSCC, particularly in HPV-related oropharyngeal cases[252], by facilitating HPV-related carcinogenesis in an immune-privileged site[253]. Furthermore, an investigation into a panel of serum cytokine and chemokine markers revealed significantly decreased IFN-γ in HNSCC patients[254], which may be caused by inhibition of T-cell regulation from increased expression of PD-1:PD-L1. Immune checkpoint blockade through a monoclonal antibody that inhibits the PD-1 receptor has the potential to play a big role in future therapy, because initiation of anti-tumour response is observed on PD-1 blockade in animal studies[255].
Personalised therapy may be possible with robust biomarker panels and it is detailed molecular analysis, such as DNA profiling[204], that may guide biomarker development. Limited success of individual markers to predict tumour behaviour has led to attempts to classify biomarker “signatures” such as panels of RNA or protein expression alterations[256]. It is possible that miRNA panels associated with HNSCC subsets may also act as biomarkers to improve diagnosis and management[257]. Some studies have investigated panels of predictive biomarkers in both HPV-related oropharyngeal SCC[258] and non-HPV related oropharyngeal SCC[259], however few of these are validated[239].
Research on biomarkers in HNSCC is a rapidly expanding field, with new potential markers that may provide valid therapeutic targets[260], however it is difficult to demonstrate clinical utility without well designed biomarkers or panels undergoing rigorous assessment in clinical trials. Hence many questions remain, with HPV infection as yet not formally validated as a predictive biomarker for any specific treatment modality or agent[256]. More practical diagnostics could be achieved through serum[137,239] or radiological[256] biomarkers, however clinical utility of these remains to be proven. There is no standardisation of detection and when p16 expression is used as a marker for HPV infection, approximately 10% of cases may be false positives[167], such that a combination of p16 overexpression with HPV DNA positivity may currently represent the most practical investigation for biologically relevant HPV infection[200] and this has been shown to be the most relevant group in terms of prognosis[261]. The relevance of infection in head and neck cancer outside the oropharynx is unestablished and identification of robust fingerprints of HPV carcinogenesis will help to improve the estimate of HPV-related non-oropharyngeal HNSCC.
HNSCC has a huge impact upon quality of life and longevity. Improvements in clinical outcome have been forthcoming through advancements in surgical technique, radiation oncology and emerging chemotherapeutic and biologic agents, however, despite a multidisciplinary team approach, treatments remain complex with an associated high morbidity and only two new treatments (EGFR antibodies and robotic surgery) have been approved in the past 30 years[262].
HPV-related oropharyngeal SCC, distinct from other HNSCC[39,263], generally presents with a more advanced clinical stage, with a higher nodal category[248,264], despite lower tumour extent[133,264] and have different tendencies for extracapsular spread and perineural invasion[265]. These HPV-related tumours may even be clinically occult, but often present with early lymph node metastases[14,266], which can be confused with branchial cleft cysts[267]. However, tonsil SCCs are long known to present with early lymph node metastases[268] and it may be that the characteristics of the affected site itself facilitate early spread or else potentially the depth of invasion[266].
As stated above, these patients tend to be younger and are less likely to have significant exposure to tobacco and alcohol. Despite more advanced presentation, improved survival, consistently higher than 30%[269], is evident in HPV-related oropharyngeal SCC[66,266,270,271], irrespective of treatment modality[133,220,272-276]. It has been suggested, therefore, that the current classification system for HNSCCs be altered to reflect the different status of HPV-related HNSCCs[273].
Detection of biologically relevant HPV infection is best accomplished using HPV E6 and E7 mRNA, however p16 in combination with HPV DNA correlates well and can be a practical alternative[277]. Studies have also shown an improved response to therapy from HPV-related HNSCCs[16,133,278-280]. As a result of this, it is possible that de-escalation of therapy would be appropriate for these tumours to improve associated morbidity and quality of life. Considering this, there are currently a number ongoing trials. A summary of some of these trials is shown in Table 1.
| Trial | Phase | Inclusion | Arm 1 | Arm 2 | Outcomes |
| RTOG 1016 | III | p16 positive locally advanced oropharyngeal SCC | Radiation and concurrent chemotherapy | Radiation and concurrent cetuximab | Survival, toxicity, locoregional recurrence and quality of life |
| ECOG E1308 | II | Stage III-IVa HPV positive oropharyngeal SCC | Complete response to induction chemotherapy and reduced dose radiation with concurrent cetuximab | Incomplete response to induction chemotherapy and standard dose radiation with concurrent cetuximab | Survival, toxicity, response, quality of life and biomarker correlation |
| De-ESCALaTE HPV | III | StageIII-IVa HPV positive oropharyngeal SCC | Cetuximab and concurrent radiotherapy | standard concurrent cisplatin and chemoradiotherapy | Morbidity, quality of life, cost, survival and recurrence |
| QUARTERBACK | III | Locally advanced HPV-16 positive oropharyngeal, unknown primary or nasopharyngeal SCC showing complete or partial response to induction therapy | Reduced dose radiation with cetuximab and chemotherapy | Standard dose radiation with chemotherapy | Survival, locoregional control, toxicity and biomarker correlation. |
| LCCC 1120 | II | HPV positive and/or p16 positive low-risk oropharyngeal SCC | Decreased dose of radiation and chemotherapy | Standard radiation and chemotherapy | Pathological response rate, locoregional control, survival and quality of life |
| NCT01221753 | II | Locally advanced HPV positive oropharyngeal SCC | Docetaxel/cisplatin/5-fluorouracil (TPF) induction chemotherapy followed by concurrent chemoradiation using a modified radiation dose | N/A | Locoregional control, survival and toxicity |
| SIRS | II | Early to mid-stage HPV positive oropharyngeal SCC who receive transoral robotic surgery plus a neck dissection, where clinically indicated | Observation only | Low dose postoperative radiation only Arm 3: Chemoradiation | Rates of locoregional control, overall survival and use of salvage chemoradiation in the observation group |
| TROG 12.01 | III | HPV positive oropharyngeal SCC | Radiation and cetuximab | Radiation and cisplatin | Symptoms severity, swallowing, quality of life, toxicity, survival, locoregional recurrence |
| ADEPT | III | p16 positive oropharyngeal SCC that has undergone transoral resection with negative margins | Postoperative radiation alone | Postoperative radiation with cisplatin | Survival, locoregional control, toxicity and quality of life |
| NCT01088802 | I/II | HPV positive T1-3 oropharyngeal SCC | De-escalated radiation from 70 Gy to 63 Gy with concurrent chemotherapy | De-escalated radiation from 58.1 Gy to 50.75 Gy with concurrent chemotherapy | Toxicity, quality of life and adverse events |
| ECOG E3311 | II | Stage III-IVa HPV positive oropharyngeal SCC after transoral surgery and neck dissection with negative margins, no extracapsular spread and less than 4 lymph nodes involved | Transoral surgery with standard radiation | Transoral surgery with low-dose radiation | Survival, surgical complications, toxicity and swallowing |
There have been conflicting reports on the benefit of cetuximab in HPV-related oropharyngeal SCC. While subset analysis in one study suggests improved survival for oropharyngeal SCCs in the cetuximab group (although not necessarily HPV-related)[281], others including the RTOG 0522 and SPECRUM trials disagree[269,282]. Preclinical investigation on treatment effects are limited by the sparse number of HPV-related HNSCC cell lines available.
While organ-preservation trials have led to primary chemoradiotherapy superseding surgical management in HNSCC, there has been renewed interest in transoral techniques for oropharyngeal SCC, particularly with the introduction of robotic surgery. Equivalent early oncologic outcomes to chemoradiotherapy and improved functional outcomes are promising[283]. Some trials involving transoral surgery are shown in Table 1.
Therapeutic vaccines are novel strategies aimed at improving the T-cell mediated immune response to HPV-related SCCs. Recent phase I and II clinical trials, some in combination with chemotherapy to boost effectiveness, are investigating these[269,284].
There is currently no single standardized treatment for oropharyngeal SCCs, but before recommended management strategies are altered, results from randomized controlled trials are needed to assess the efficacy of the different treatment modalities available for both HPV-positive and HPV-negative oropharyngeal SCC[285], although recruitment of sufficient numbers remains difficult[265].
Induction of HPV-specific immune responses by prophylactic vaccination with recombinant HPV virus-like particles is likely the key to successful prevention of persistent HPV infection and the subsequent consequences. As such, bivalent and quadrivalent vaccines are now widely available and have shown efficacy in prevention of anal, cervical, vaginal, and vulvar pre-cancers in unexposed individuals[94,286,287]. Unfortunately, present vaccines are only proven to be effective if given before genotype-specific infection is established[288], duration of protection remains unclear and cost is high. Given the high specificity of oropharyngeal cases linked to HPV-16, it is unlikely that other genotypes would replace HPV-16, particularly in view of evidence for induction of cross-genotype immunity with genotype-specific immunisation[289].
In relation to the oropharynx, animal model investigation has revealed reduction in development of HPV-related oral lesions in immunised cases[290]. Recently, an IARC-led study established that a bivalent vaccine used for cervical cancer prevention also reduced oral infections with HPV 16 and 18 by 93.3%[291]. While oral HPV infection is a risk factor for development of HPV-related oropharyngeal SCC, pathogenesis is unclear and the lack of an obvious HPV-related precancerous stage does not facilitate screening and makes evaluation of vaccine effectiveness difficult. Accurate estimates of HPV-related oropharyngeal SCC will help determine the potential role of prophylactic vaccination. It is likely that the effects of vaccination on oropharyngeal SCC will only be revealed over time through longitudinal studies on incidence before and after vaccine introduction.
Treatment of HPV-related oropharyngeal SCC is currently varied geographically depending on tumour stage, patient status including age and co-morbidities, facilities available and HPV or p16 status. There remains uncertainty regarding vaccination, cetuximab and de-escalation of therapy, which will be made clearer through current prospective trials, leading to better delineation of therapy for HNSCC subsets. Accurate assessment for biologically relevant HPV will be critical to improvement in treatment approaches.
HPV has been established beyond doubt as a causative agent in oropharyngeal SCC and biologically active HPV can act as a prognosticator with better overall survival than HPV-negative HNSCCs. A distinct group of younger patients with limited tobacco and alcohol exposure have emerged as characteristic of this HPV-related subset of HNSCC. However, the exact molecular mechanisms of carcinogenesis are not completely described and further studies are needed to assist development of optimal prevention and treatment modalities.
Despite the large pool of research on HPV in HNSCC, great variation exists in detection techniques. Detection of biologically relevant HPV infection will be important for clinical trial design. Also, biomarker discovery will be important not only to identify specific SCC subsets, including those that are HPV-related, to allow for individualised treatment strategies aimed at decreasing morbidity, but also to clarify the role of HPV in non-oropharyngeal sites.
With stored tissue available from the SEER database in only 271 patients[265], there needs to be greater cooperation between institutions to improve research into understanding this disease. Nevertheless, it is likely that the key approach in future will be prevention and so further studies in prophylactic vaccination, specifically in relation to oropharyngeal SCC, are needed.
P- Reviewers: Chen GS, Mandic R, Tian YP, Yeudall WA S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA. Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer. 2007;43:415-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | O’Regan EM, Toner ME, Smyth PC, Finn SP, Timon C, Cahill S, Flavin R, O’Leary JJ, Sheils O. Distinct array comparative genomic hybridization profiles in oral squamous cell carcinoma occurring in young patients. Head Neck. 2006;28:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1-30. [PubMed] |
| 5. | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 1995;64:1-378. [PubMed] |
| 6. | Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 663] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 9. | Aldabagh B, Angeles JG, Cardones AR, Arron ST. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1970] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 11. | Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, Stern PL, Stanley M, Arbyn M, Poljak M. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31 Suppl 7:H1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 12. | Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418-424. [PubMed] |
| 13. | Löning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, zur Hausen H. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol. 1985;84:417-420. [PubMed] |
| 14. | Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer’s tonsillar ring. Cancer. 1997;79:595-604. [PubMed] |
| 15. | Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, Conradt C, Klein W, Helbig M, Dietz A, Weidauer H. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58:5-13. [PubMed] |
| 16. | Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709-720. [PubMed] |
| 17. | El-Mofty SK. Human papillomavirus (HPV) related carcinomas of the upper aerodigestive tract. Head Neck Pathol. 2007;1:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22:1125-1142, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Cohan DM, Popat S, Kaplan SE, Rigual N, Loree T, Hicks WL. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg. 2009;17:88-94. [PubMed] |
| 21. | Slebos RJ, Yi Y, Ely K, Carter J, Evjen A, Zhang X, Shyr Y, Murphy BM, Cmelak AJ, Burkey BB. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007;67:4605-4619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Braakhuis BJ, Snijders PJ, Keune WJ, Meijer CJ, Ruijter-Schippers HJ, Leemans CR, Brakenhoff RH. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96:998-1006. [PubMed] |
| 24. | Li G, Huang Z, Chen X, Wei Q. Role of human papillomavirus and cell cycle-related variants in squamous cell carcinoma of the oropharynx. J Biomed Res. 2010;24:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694-5699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 236] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Kreimer AR, Clifford GM, Snijders PJ, Castellsagué X, Meijer CJ, Pawlita M, Viscidi R, Herrero R, Franceschi S. HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int J Cancer. 2005;115:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1930] [Cited by in RCA: 1834] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 28. | Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32:1044-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Jo VY, Mills SE, Stoler MH, Stelow EB. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Carpenter DH, El-Mofty SK, Lewis JS. Undifferentiated carcinoma of the oropharynx: a human papillomavirus-associated tumor with a favorable prognosis. Mod Pathol. 2011;24:1306-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Singhi AD, Stelow EB, Mills SE, Westra WH. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ, Lewis JS. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | O’Duffy F, O’Dwyer TP. The growing epidemic of HPV associated oropharyngeal malignancy. Ir Med J. 2012;105:101-102. [PubMed] |
| 37. | Näsman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M, Lindquist D. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 38. | Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 567] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 39. | Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1350] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 40. | Panwar A, Batra R, Lydiatt WM, Ganti AK. Human papilloma virus positive oropharyngeal squamous cell carcinoma: a growing epidemic. Cancer Treat Rev. 2014;40:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6 Suppl 1:S16-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6 Suppl 1:S104-S120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24 Suppl 3:S3/1-S310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 741] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 44. | de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2081] [Cited by in RCA: 2082] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 45. | Conway MJ, Meyers C. Replication and assembly of human papillomaviruses. J Dent Res. 2009;88:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Qmichou Z, Khyatti M, Berraho M, Ennaji MM, Benbacer L, Nejjari C, Benjaafar N, Benider A, Attaleb M, El Mzibri M. Analysis of mutations in the E6 oncogene of human papillomavirus 16 in cervical cancer isolates from Moroccan women. BMC Infect Dis. 2013;13:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Pande S, Jain N, Prusty BK, Bhambhani S, Gupta S, Sharma R, Batra S, Das BC. Human papillomavirus type 16 variant analysis of E6, E7, and L1 genes and long control region in biopsy samples from cervical cancer patients in north India. J Clin Microbiol. 2008;46:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Xi LF, Koutsky LA, Hildesheim A, Galloway DA, Wheeler CM, Winer RL, Ho J, Kiviat NB. Risk for high-grade cervical intraepithelial neoplasia associated with variants of human papillomavirus types 16 and 18. Cancer Epidemiol Biomarkers Prev. 2007;16:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Tornesello ML, Duraturo ML, Salatiello I, Buonaguro L, Losito S, Botti G, Stellato G, Greggi S, Piccoli R, Pilotti S. Analysis of human papillomavirus type-16 variants in Italian women with cervical intraepithelial neoplasia and cervical cancer. J Med Virol. 2004;74:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Pista A, Oliveira A, Barateiro A, Costa H, Verdasca N, Paixão MT. Molecular variants of human papillomavirus type 16 and 18 and risk for cervical neoplasia in Portugal. J Med Virol. 2007;79:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Cornet I, Gheit T, Clifford GM, Combes JD, Dalstein V, Franceschi S, Tommasino M, Clavel C. Human papillomavirus type 16 E6 variants in France and risk of viral persistence. Infect Agent Cancer. 2013;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbrough WG, Issaeva N. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res. 2012;18:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1713] [Article Influence: 131.8] [Reference Citation Analysis (1)] |
| 54. | Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 55. | Gillison ML, Castellsagué X, Chaturvedi A, Goodman MT, Snijders P, Tommasino M, Arbyn M, Franceschi S. Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 56. | Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 57. | de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1892] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 58. | St Guily JL, Jacquard AC, Prétet JL, Haesebaert J, Beby-Defaux A, Clavel C, Agius G, Birembaut P, Okaïs C, Léocmach Y. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France--The EDiTH VI study. J Clin Virol. 2011;51:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2814] [Cited by in RCA: 2755] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 60. | Kero K, Rautava J, Syrjänen K, Grenman S, Syrjänen S. Oral mucosa as a reservoir of human papillomavirus: point prevalence, genotype distribution, and incident infections among males in a 7-year prospective study. Eur Urol. 2012;62:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | St Guily JL, Clavel C, Okaïs C, Prétet JL, Beby-Defaux A, Agius G, Birembaut P, Jacquard AC, Léocmach Y, Soubeyrand B. Human papillomavirus genotype distribution in tonsil cancers. Head Neck Oncol. 2011;3:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 773] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 63. | Kreimer AR, Bhatia RK, Messeguer AL, González P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010;37:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 64. | Beachler DC, Weber KM, Margolick JB, Strickler HD, Cranston RD, Burk RD, Wiley DJ, Minkoff H, Reddy S, Stammer EE. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 65. | Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 1473] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 66. | Chien CY, Su CY, Fang FM, Huang HY, Chuang HC, Chen CM, Huang CC. Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol. 2008;44:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 67. | Ribeiro KB, Levi JE, Pawlita M, Koifman S, Matos E, Eluf-Neto J, Wunsch-Filho V, Curado MP, Shangina O, Zaridze D. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011;40:489-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Ernster JA, Sciotto CG, O’Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117:2115-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Auluck A, Hislop G, Bajdik C, Poh C, Zhang L, Rosin M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (HPV)-related and HPV-unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116:2635-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Hocking JS, Stein A, Conway EL, Regan D, Grulich A, Law M, Brotherton JM. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104:886-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Lee YC, Zugna D, Richiardi L, Merletti F, Marron M, Ahrens W, Pohlabeln H, Lagiou P, Trichopoulos D, Agudo A. Smoking addiction and the risk of upper-aerodigestive-tract cancer in a multicenter case-control study. Int J Cancer. 2013;133:2688-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Guha N, Boffetta P, Wünsch Filho V, Eluf Neto J, Shangina O, Zaridze D, Curado MP, Koifman S, Matos E, Menezes A. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case-control studies. Am J Epidemiol. 2007;166:1159-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 73. | Talamini R, Vaccarella S, Barbone F, Tavani A, La Vecchia C, Herrero R, Muñoz N, Franceschi S. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer. 2000;83:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E, Barzan L, La Vecchia C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br J Cancer. 1999;80:614-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Fioretti F, Bosetti C, Tavani A, Franceschi S, La Vecchia C. Risk factors for oral and pharyngeal cancer in never smokers. Oral Oncol. 1999;35:375-378. [PubMed] |
| 76. | Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 79. | Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdhan A, Shah JP, Singh B. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106-112. [PubMed] |
| 80. | Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, Huvos AG, Giampietro P, Levran O, Pujara K. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95:1718-1721. [PubMed] |
| 81. | Toner M, O’Regan EM. Head and neck squamous cell carcinoma in the young: a spectrum or a distinct group? Part 2. Head Neck Pathol. 2009;3:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | O’Regan EM, Toner ME, Finn SP, Fan CY, Ring M, Hagmar B, Timon C, Smyth P, Cahill S, Flavin R. p16(INK4A) genetic and epigenetic profiles differ in relation to age and site in head and neck squamous cell carcinomas. Hum Pathol. 2008;39:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, Haugen TH, Turek LP. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 84. | El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463-1470. [PubMed] |
| 85. | Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550-4559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 985] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 86. | Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1167] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 87. | Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1093] [Cited by in RCA: 1110] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 88. | Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, Turek LP, Haugen TH. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 89. | Smith EM, Ritchie JM, Pawlita M, Rubenstein LM, Haugen TH, Turek LP, Hamsikova E. Human papillomavirus seropositivity and risks of head and neck cancer. Int J Cancer. 2007;120:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Schwartz SM, Daling JR, Doody DR, Wipf GC, Carter JJ, Madeleine MM, Mao EJ, Fitzgibbons ED, Huang S, Beckmann AM. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626-1636. [PubMed] |
| 91. | Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772-1783. [PubMed] |
| 92. | Martín-Hernán F, Sánchez-Hernández JG, Cano J, Campo J, del Romero J. Oral cancer, HPV infection and evidence of sexual transmission. Med Oral Patol Oral Cir Bucal. 2013;18:e439-e444. [PubMed] |
| 93. | Syrjanen S, Termine N, Capra G, Paderni C, Panzarella V, Campisi G. Oral HPV infection: current strategies for prevention and therapy. Curr Pharm Des. 2012;18:5452-5469. [PubMed] |
| 94. | D’Souza G, Dempsey A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med. 2011;53 Suppl 1:S5-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 95. | D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 96. | Fakhry C, D’souza G, Sugar E, Weber K, Goshu E, Minkoff H, Wright R, Seaberg E, Gillison M. Relationship between prevalent oral and cervical human papillomavirus infections in human immunodeficiency virus-positive and -negative women. J Clin Microbiol. 2006;44:4479-4485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Termine N, Giovannelli L, Matranga D, Caleca MP, Bellavia C, Perino A, Campisi G. Oral human papillomavirus infection in women with cervical HPV infection: new data from an Italian cohort and a metanalysis of the literature. Oral Oncol. 2011;47:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, Shah KV, Gillison ML. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Andrews E, Shores C, Hayes DN, Couch M, Southerland J, Morris D, Seaman WT, Webster-Cyriaque J. Concurrent human papillomavirus-associated tonsillar carcinoma in 2 couples. J Infect Dis. 2009;200:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Vogt SL, Gravitt PE, Martinson NA, Hoffmann J, D’Souza G. Concordant Oral-Genital HPV Infection in South Africa Couples: Evidence for Transmission. Front Oncol. 2013;3:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Mbulawa ZZ, Johnson LF, Marais DJ, Coetzee D, Williamson AL. Risk factors for oral human papillomavirus in heterosexual couples in an African setting. J Infect. 2014;68:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Beder Ribeiro CM, Ferrer I, Santos de Farias AB, Fonseca DD, Morais Silva IH, Monteiro Gueiros LA, Carvalho AT, Porter SR, Leao JC. Oral and genital HPV genotypic concordance between sexual partners. Clin Oral Investig. 2014;18:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Pickard RK, Xiao W, Broutian TR, He X, Gillison ML. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex Transm Dis. 2012;39:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 104. | Rautava J, Syrjänen S. Human papillomavirus infections in the oral mucosa. J Am Dent Assoc. 2011;142:905-914. [PubMed] |
| 105. | Rioux M, Garland A, Webster D, Reardon E. HPV positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013;42:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Gillison ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health. 2008;43:S52-S60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 107. | Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, Perez-Ordonez B, Jordan RC, Gillison ML. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 108. | D’Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, Anastos K, Palefsky JM, Gillison ML. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 109. | Steinau M, Hariri S, Gillison ML, Broutian TR, Dunne EF, Tong ZY, Markowitz LE, Unger ER. Prevalence of Cervical and Oral Human Papillomavirus Infections Among US Women. J Infect Dis. 2014;209:1739-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, Hildesheim A, Villa LL, Salmerón JJ, Lazcano-Ponce E. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382:877-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 111. | Appleby P, Beral V, Berrington de González A, Colin D, Franceschi S, Goodill A, Green J, Peto J, Plummer M, Sweetland S. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118:1481-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 112. | Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805-813. [PubMed] |
| 113. | Sinha P, Logan HL, Mendenhall WM. Human papillomavirus, smoking, and head and neck cancer. Am J Otolaryngol. 2012;33:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 114. | Marks MA, Chaturvedi AK, Kelsey K, Straif K, Berthiller J, Schwartz SM, Smith E, Wyss A, Brennan P, Olshan AF. Association of marijuana smoking with oropharyngeal and oral tongue cancers: pooled analysis from the INHANCE consortium. Cancer Epidemiol Biomarkers Prev. 2014;23:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 115. | American Lung Association - Epidemiology and Statistics Unit, Research and Program Services. Trends in Tobacco Use. Washington, DC: American Lung Association 2011; . |
| 116. | Colevas AD. Population-based evaluation of incidence trends in oropharyngeal cancer focusing on socioeconomic status, sex, and race/ethnicity. Head Neck. 2014;36:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2111] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 118. | Shatalova EG, Klein-Szanto AJ, Devarajan K, Cukierman E, Clapper ML. Estrogen and cytochrome P450 1B1 contribute to both early- and late-stage head and neck carcinogenesis. Cancer Prev Res (Phila). 2011;4:107-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009;200:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 120. | Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn LJ, Sherman ME, Xhenseval V. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 121. | Miller CS, White DK. Human papillomavirus expression in oral mucosa, premalignant conditions, and squamous cell carcinoma: a retrospective review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:57-68. [PubMed] |
| 122. | Kreimer AR, Villa A, Nyitray AG, Abrahamsen M, Papenfuss M, Smith D, Hildesheim A, Villa LL, Lazcano-Ponce E, Giuliano AR. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev. 2011;20:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 123. | Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 124. | Gillison ML. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr Opin Oncol. 2009;21:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 125. | Beachler DC, D’Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis. 2013;208:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 126. | García-Piñeres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, Ramírez M, Villegas M, Schiffman M, Rodríguez AC. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070-11076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 127. | Keller MJ, Burk RD, Xie X, Anastos K, Massad LS, Minkoff H, Xue X, D’Souza G, Watts DH, Levine AM. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012;308:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Branca M, Garbuglia AR, Benedetto A, Cappiello T, Leoncini L, Migliore G, Agarossi A, Syrjänen K. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003;14:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258-J265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 655] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 130. | Simen-Kapeu A, Kataja V, Yliskoski M, Syrjänen K, Dillner J, Koskela P, Paavonen J, Lehtinen M. Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scand J Infect Dis. 2008;40:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 131. | O’Hanlon S, Forster DP, Lowry RJ. Oral cancer in the North-East of England: incidence, mortality trends and the link with material deprivation. Community Dent Oral Epidemiol. 1997;25:371-376. [PubMed] |
| 132. | Boscolo-Rizzo P, Del Mistro A, Bussu F, Lupato V, Baboci L, Almadori G, DA Mosto MC, Paludetti G. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33:77-87. [PubMed] |
| 133. | Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2091] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 134. | Jiron J, Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, Kato I. Racial disparities in Human Papillomavirus (HPV) associated head and neck cancer. Am J Otolaryngol. 2014;35:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 135. | Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, Strome SE, Haddad RI, Patel SS, Cambell EV. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila). 2009;2:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 136. | Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 607] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 137. | Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, Tjønneland A, Overvad K, Quirós JR, González CA. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 138. | Toner M, O’Regan EM. Head and neck squamous cell carcinoma in the young: a spectrum or a distinct group? Part 1. Head Neck Pathol. 2009;3:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 139. | Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, Butterfoss D, Xiang D, Zanation A, Yin X. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489-500. [PubMed] |
| 140. | Walter V, Yin X, Wilkerson MD, Cabanski CR, Zhao N, Du Y, Ang MK, Hayward MC, Salazar AH, Hoadley KA. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8:e56823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 141. | Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1368] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 142. | Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2034] [Cited by in RCA: 1964] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 143. | Zhang XC, Xu C, Mitchell RM, Zhang B, Zhao D, Li Y, Huang X, Fan W, Wang H, Lerma LA. Tumor evolution and intratumor heterogeneity of an oropharyngeal squamous cell carcinoma revealed by whole-genome sequencing. Neoplasia. 2013;15:1371-1378. [PubMed] |
| 144. | Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013;3:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 472] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 145. | Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012;122:1951-1957. [PubMed] |
| 146. | Chinn SB, Darr OA, Owen JH, Bellile E, McHugh JB, Spector ME, Papagerakis SM, Chepeha DB, Bradford CR, Carey TE. Cancer stem cells: Mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck. 2014;Jan 10; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Chinn SB, Darr OA, Peters RD, Prince ME. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Front Endocrinol (Lausanne). 2012;3:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 148. | Rastogi P. Emergence of cancer stem cells in head and neck squamous cell carcinoma: A therapeutic insight with literature review. Dent Res J (Isfahan). 2012;9:239-244. [PubMed] |
| 149. | Bhaijee F, Pepper DJ, Pitman KT, Bell D. Cancer stem cells in head and neck squamous cell carcinoma: a review of current knowledge and future applications. Head Neck. 2012;34:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 150. | Iglesias-Bartolome R, Martin D, Gutkind JS. Exploiting the head and neck cancer oncogenome: widespread PI3K-mTOR pathway alterations and novel molecular targets. Cancer Discov. 2013;3:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 151. | Urashima M, Hama T, Suda T, Suzuki Y, Ikegami M, Sakanashi C, Akutsu T, Amagaya S, Horiuchi K, Imai Y. Distinct effects of alcohol consumption and smoking on genetic alterations in head and neck carcinoma. PLoS One. 2013;8:e80828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | SLAUGHTER DP, SOUTHWICK HW, SMEJKAL W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [PubMed] |
| 153. | Graveland AP, Bremmer JF, de Maaker M, Brink A, Cobussen P, Zwart M, Braakhuis BJ, Bloemena E, van der Waal I, Leemans CR. Molecular screening of oral precancer. Oral Oncol. 2013;49:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 154. | Lucio M, Andrea G, Bartolomeo GD, Fabio C, Dora S. Between-lesion discrepancies in terms of dysplasia, cell turnover and diagnosis in patients with multiple potentially malignant oral lesions. Open Dent J. 2013;7:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | Sudbø J, Bryne M, Johannessen AC, Kildal W, Danielsen HE, Reith A. Comparison of histological grading and large-scale genomic status (DNA ploidy) as prognostic tools in oral dysplasia. J Pathol. 2001;194:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 156. | Sudbø J, Kildal W, Johannessen AC, Koppang HS, Sudbø A, Danielsen HE, Risberg B, Reith A. Gross genomic aberrations in precancers: clinical implications of a long-term follow-up study in oral erythroplakias. J Clin Oncol. 2002;20:456-462. [PubMed] |
| 157. | Rietbergen MM, Braakhuis BJ, Moukhtari N, Bloemena E, Brink A, Sie D, Ylstra B, Baatenburg de Jong RJ, Snijders PJ, Brakenhoff RH. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J Oral Pathol Med. 2014;43:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 158. | Jain KS, Sikora AG, Baxi SS, Morris LG. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. 2013;119:1832-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 159. | Smeets SJ, Braakhuis BJ, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, Meijer GA, Leemans CR, Brakenhoff RH. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25:2558-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 160. | Wilting SM, Smeets SJ, Snijders PJ, van Wieringen WN, van de Wiel MA, Meijer GA, Ylstra B, Leemans CR, Meijer CJ, Brakenhoff RH. Genomic profiling identifies common HPV-associated chromosomal alterations in squamous cell carcinomas of cervix and head and neck. BMC Med Genomics. 2009;2:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 161. | Perrone F, Suardi S, Pastore E, Casieri P, Orsenigo M, Caramuta S, Dagrada G, Losa M, Licitra L, Bossi P. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2006;12:6643-6651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 162. | Strati K, Lambert PF. Human papillomavirus association with head and neck cancers: understanding virus biology and using it in the development of cancer diagnostics. Expert Opin Med Diagn. 2008;2:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 163. | Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond). 2006;110:525-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 645] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 164. | Heffernan CB, O’Neill JP, Timon C. Oncogenic impact of human papilloma virus in head and neck cancer. J Laryngol Otol. 2010;124:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 165. | Kim SH, Koo BS, Kang S, Park K, Kim H, Lee KR, Lee MJ, Kim JM, Choi EC, Cho NH. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 166. | Thavaraj S, Stokes A, Mazuno K, Henley-Smith R, Suh YE, Paleri V, Tavassoli M, Odell E, Robinson M. Patients with HPV-related tonsil squamous cell carcinoma rarely harbour oncogenic HPV infection at other pharyngeal sites. Oral Oncol. 2014;50:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 167. | Rautava J, Syrjänen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6 Suppl 1:S3-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 168. | Fujimura Y, Takeda M, Ikai H, Haruma K, Akisada T, Harada T, Sakai T, Ohuchi M. The role of M cells of human nasopharyngeal lymphoid tissue in influenza virus sampling. Virchows Arch. 2004;444:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 169. | Moutsopoulos NM, Vázquez N, Greenwell-Wild T, Ecevit I, Horn J, Orenstein J, Wahl SM. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J Leukoc Biol. 2006;80:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 170. | Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, Ramaekers FC, Hopman AH, Manni JJ. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5-8. Int J Cancer. 2003;107:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 171. | Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD, Xian W. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci USA. 2012;109:10516-10521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 172. | Herfs M, Vargas SO, Yamamoto Y, Howitt BE, Nucci MR, Hornick JL, McKeon FD, Xian W, Crum CP. A novel blueprint for ‘top down’ differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 173. | Herfs M, Parra-Herran C, Howitt BE, Laury AR, Nucci MR, Feldman S, Jimenez CA, McKeon FD, Xian W, Crum CP. Cervical squamocolumnar junction-specific markers define distinct, clinically relevant subsets of low-grade squamous intraepithelial lesions. Am J Surg Pathol. 2013;37:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 174. | Brook I. The clinical microbiology of Waldeyer’s ring. Otolaryngol Clin North Am. 1987;20:259-272. [PubMed] |
| 175. | Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008;35:519-536; vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 176. | Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Désy M, Rohan TE. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 177. | Lace MJ, Anson JR, Klussmann JP, Wang DH, Smith EM, Haugen TH, Turek LP. Human papillomavirus type 16 (HPV-16) genomes integrated in head and neck cancers and in HPV-16-immortalized human keratinocyte clones express chimeric virus-cell mRNAs similar to those found in cervical cancers. J Virol. 2011;85:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 178. | Park IS, Chang X, Loyo M, Wu G, Chuang A, Kim MS, Chae YK, Lyford-Pike S, Westra WH, Saunders JR. Characterization of the methylation patterns in human papillomavirus type 16 viral DNA in head and neck cancers. Cancer Prev Res (Phila). 2011;4:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 179. | Woo SB, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26:1288-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 180. | Kreimer AR, Chaturvedi AK. HPV-associated Oropharyngeal Cancers--Are They Preventable? Cancer Prev Res (Phila). 2011;4:1346-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 181. | Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila). 2011;4:1378-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 182. | Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101:412-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 183. | Rampias T, Sasaki C, Psyrri A. Molecular mechanisms of HPV induced carcinogenesis in head and neck. Oral Oncol. 2014;50:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 184. | Oh JE, Kim JO, Shin JY, Zhang XH, Won HS, Chun SH, Jung CK, Park WS, Nam SW, Eun JW. Molecular genetic characterization of p53 mutated oropharyngeal squamous cell carcinoma cells transformed with human papillomavirus E6 and E7 oncogenes. Int J Oncol. 2013;43:383-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Duensing S, Münger K. Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins. Int J Cancer. 2004;109:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 186. | Gao G, Johnson SH, Kasperbauer JL, Eckloff BW, Tombers NM, Vasmatzis G, Smith DI. Mate pair sequencing of oropharyngeal squamous cell carcinomas reveals that HPV integration occurs much less frequently than in cervical cancer. J Clin Virol. 2014;59:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 187. | Olthof NC, Speel EJ, Kolligs J, Haesevoets A, Henfling M, Ramaekers FC, Preuss SF, Drebber U, Wieland U, Silling S. Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS One. 2014;9:e88718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 188. | Pannone G, Rodolico V, Santoro A, Lo Muzio L, Franco R, Botti G, Aquino G, Pedicillo MC, Cagiano S, Campisi G. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer. 2012;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 189. | Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, Pawlita M. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol. 2013;51:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 190. | Holzinger D, Schmitt M, Dyckhoff G, Benner A, Pawlita M, Bosch FX. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res. 2012;72:4993-5003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 191. | Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, Abecassis J, Clavel C, Wasylyk B. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 192. | Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 193. | Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690-7700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 322] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 194. | McMurray HR, McCance DJ. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J Virol. 2003;77:9852-9861. [PubMed] |
| 195. | Mischo A, Ohlenschläger O, Hortschansky P, Ramachandran R, Görlach M. Structural insights into a wildtype domain of the oncoprotein E6 and its interaction with a PDZ domain. PLoS One. 2013;8:e62584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 196. | Liu X, Clements A, Zhao K, Marmorstein R. Structure of the human Papillomavirus E7 oncoprotein and its mechanism for inactivation of the retinoblastoma tumor suppressor. J Biol Chem. 2006;281:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 197. | Bose S, Evans H, Lantzy L, Scharre K, Youssef E. p16(INK4A) is a surrogate biomarker for a subset of human papilloma virus-associated dysplasias of the uterine cervix as determined on the Pap smear. Diagn Cytopathol. 2005;32:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 198. | Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 199. | El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 200. | Wang H, Sun R, Lin H, Hu WH. P16INK4A as a surrogate biomarker for human papillomavirus-associated oropharyngeal carcinoma: consideration of some aspects. Cancer Sci. 2013;104:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 201. | Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1211] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 202. | Jabbar S, Strati K, Shin MK, Pitot HC, Lambert PF. Human papillomavirus type 16 E6 and E7 oncoproteins act synergistically to cause head and neck cancer in mice. Virology. 2010;407:60-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Schlecht NF, Burk RD, Adrien L, Dunne A, Kawachi N, Sarta C, Chen Q, Brandwein-Gensler M, Prystowsky MB, Childs G. Gene expression profiles in HPV-infected head and neck cancer. J Pathol. 2007;213:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 204. | Jenkins G, O’Byrne KJ, Panizza B, Richard DJ. Genome Stability Pathways in Head and Neck Cancers. Int J Genomics. 2013;2013:464720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 205. | Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA. 2000;97:10002-10007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 331] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 206. | Patel D, Incassati A, Wang N, McCance DJ. Human papillomavirus type 16 E6 and E7 cause polyploidy in human keratinocytes and up-regulation of G2-M-phase proteins. Cancer Res. 2004;64:1299-1306. [PubMed] |
| 207. | Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62:7075-7082. [PubMed] |
| 208. | Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, Rocco JW, Teknos TN, Kumar B, Wangsa D. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014;24:185-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 209. | Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci U S A. 2006;103:14152-14157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 210. | Sartor MA, Dolinoy DC, Jones TR, Colacino JA, Prince ME, Carey TE, Rozek LS. Genome-wide methylation and expression differences in HPV(+) and HPV(-) squamous cell carcinoma cell lines are consistent with divergent mechanisms of carcinogenesis. Epigenetics. 2011;6:777-787. [PubMed] |
| 211. | Lajer CB, Garnæs E, Friis-Hansen L, Norrild B, Therkildsen MH, Glud M, Rossing M, Lajer H, Svane D, Skotte L. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012;106:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 212. | Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, Zhao M, Ortega Alves MV, Chang K, Drummond J. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 213. | Hafkamp HC, Manni JJ, Speel EJ. Role of human papillomavirus in the development of head and neck squamous cell carcinomas. Acta Otolaryngol. 2004;124:520-526. [PubMed] |
| 214. | Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, Clark JR, Wein RO, Grillone GA, Houseman EA. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72:5004-5013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 215. | Hirshoren N, Bulvik R, Neuman T, Rubinstein AM, Meirovitz A, Elkin M. Induction of heparanase by HPV E6 oncogene in head and neck squamous cell carcinoma. J Cell Mol Med. 2014;18:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 216. | Rainsbury JW, Ahmed W, Williams HK, Roberts S, Paleri V, Mehanna H. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck. 2013;35:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 217. | Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 541] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 218. | Duray A, Descamps G, Decaestecker C, Remmelink M, Sirtaine N, Lechien J, Ernoux-Neufcoeur P, Bletard N, Somja J, Depuydt CE. Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. Laryngoscope. 2012;122:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 219. | Ernoux-Neufcoeur P, Arafa M, Decaestecker C, Duray A, Remmelink M, Leroy X, Herfs M, Somja J, Depuydt CE, Delvenne P. Combined analysis of HPV DNA, p16, p21 and p53 to predict prognosis in patients with stage IV hypopharyngeal carcinoma. J Cancer Res Clin Oncol. 2011;137:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 220. | Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, O’Sullivan B, Kenny LM. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 591] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 221. | Klussmann JP, Gültekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ, Fuchs PG. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 222. | Lewis JS, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 223. | Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin Oncol. 2013;2:51-61. [PubMed] |
| 224. | Park K, Cho KJ, Lee M, Yoon DH, Kim J, Kim SY, Nam SY, Choi SH, Roh JL, Han MW. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol. 2013;133:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 225. | Zhao N, Ang MK, Yin XY, Patel MR, Fritchie K, Thorne L, Muldrew KL, Hayward MC, Sun W, Wilkerson MD. Different cellular p16(INK4a) localisation may signal different survival outcomes in head and neck cancer. Br J Cancer. 2012;107:482-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 226. | Klingenberg B, Hafkamp HC, Haesevoets A, Manni JJ, Slootweg PJ, Weissenborn SJ, Klussmann JP, Speel EJ. p16 INK4A overexpression is frequently detected in tumour-free tonsil tissue without association with HPV. Histopathology. 2010;56:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 227. | Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 228. | Hoffmann M, Tribius S, Quabius ES, Henry H, Pfannenschmidt S, Burkhardt C, Görögh T, Halec G, Hoffmann AS, Kahn T. HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer - how valid is p16INK4A as surrogate marker? Cancer Lett. 2012;323:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 229. | Heath S, Willis V, Allan K, Purdie K, Harwood C, Shields P, Simcock R, Williams T, Gilbert DC. Clinically significant human papilloma virus in squamous cell carcinoma of the head and neck in UK practice. Clin Oncol (R Coll Radiol). 2012;24:e18-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 230. | Hong A, Jones D, Chatfield M, Lee CS, Zhang M, Clark J, Elliott M, Harnett G, Milross C, Rose B. HPV status of oropharyngeal cancer by combination HPV DNA/p16 testing: biological relevance of discordant results. Ann Surg Oncol. 2013;20 Suppl 3:S450-S458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 231. | Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, Taube JM, Koch WM, Westra WH. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 232. | Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H, Ma XJ, Luo Y, Lewis JS, Wang X. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 233. | Schache AG, Liloglou T, Risk JM, Jones TM, Ma XJ, Wang H, Bui S, Luo Y, Sloan P, Shaw RJ. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 234. | Westra WH. Detection of human papillomavirus in clinical samples. Otolaryngol Clin North Am. 2012;45:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 235. | Rampias T, Pectasides E, Prasad M, Sasaki C, Gouveris P, Dimou A, Kountourakis P, Perisanidis C, Burtness B, Zaramboukas T. Molecular profile of head and neck squamous cell carcinomas bearing p16 high phenotype. Ann Oncol. 2013;24:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 236. | Lothaire P, de Azambuja E, Dequanter D, Lalami Y, Sotiriou C, Andry G, Castro G, Awada A. Molecular markers of head and neck squamous cell carcinoma: promising signs in need of prospective evaluation. Head Neck. 2006;28:256-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 237. | Chen YJ, Lee LY, Chao YK, Chang JT, Lu YC, Li HF, Chiu CC, Li YC, Li YL, Chiou JF. DSG3 facilitates cancer cell growth and invasion through the DSG3-plakoglobin-TCF/LEF-Myc/cyclin D1/MMP signaling pathway. PLoS One. 2013;8:e64088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 238. | Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, Chen PJ, Cheng AJ. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. 2007;26:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 239. | Langer CJ. Exploring biomarkers in head and neck cancer. Cancer. 2012;118:3882-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 240. | Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 648] [Cited by in RCA: 583] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 241. | Jedlinski A, Ansell A, Johansson AC, Roberg K. EGFR status and EGFR ligand expression influence the treatment response of head and neck cancer cell lines. J Oral Pathol Med. 2013;42:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 242. | Johansson AC, Ansell A, Jerhammar F, Lindh MB, Grénman R, Munck-Wikland E, Östman A, Roberg K. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol Cancer Res. 2012;10:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 243. | Ansell A, Jerhammar F, Ceder R, Grafström R, Grénman R, Roberg K. Matrix metalloproteinase-7 and -13 expression associate to cisplatin resistance in head and neck cancer cell lines. Oral Oncol. 2009;45:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 244. | Farnebo L, Tiefenböck K, Ansell A, Thunell LK, Garvin S, Roberg K. Strong expression of survivin is associated with positive response to radiotherapy and improved overall survival in head and neck squamous cell carcinoma patients. Int J Cancer. 2013;133:1994-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 245. | Temam S, Kawaguchi H, El-Naggar AK, Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ, Mao L. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol. 2007;25:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 246. | Hama T, Yuza Y, Saito Y, O-uchi J, Kondo S, Okabe M, Yamada H, Kato T, Moriyama H, Kurihara S. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist. 2009;14:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 247. | Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy BA, Netterville J. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170-4176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 435] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 248. | Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 249. | Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3919] [Cited by in RCA: 3653] [Article Influence: 192.3] [Reference Citation Analysis (0)] |
| 250. | Lassen P, Overgaard J, Eriksen JG. Expression of EGFR and HPV-associated p16 in oropharyngeal carcinoma: correlation and influence on prognosis after radiotherapy in the randomized DAHANCA 5 and 7 trials. Radiother Oncol. 2013;108:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 251. | Bozec A, Peyrade F, Milano G. Molecular targeted therapies in the management of head and neck squamous cell carcinoma: recent developments and perspectives. Anticancer Agents Med Chem. 2013;13:389-402. [PubMed] |
| 252. | Ukpo OC, Thorstad WL, Lewis JS. B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 253. | Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 608] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 254. | Kaskas NM, Moore-Medlin T, McClure GB, Ekshyyan O, Vanchiere JA, Nathan CA. Serum biomarkers in head and neck squamous cell cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 255. | Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 492] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 256. | Lucs AV, Saltman B, Chung CH, Steinberg BM, Schwartz DL. Opportunities and challenges facing biomarker development for personalized head and neck cancer treatment. Head Neck. 2013;35:294-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 257. | Nagadia R, Pandit P, Coman WB, Cooper-White J, Punyadeera C. miRNAs in head and neck cancer revisited. Cell Oncol (Dordr). 2013;36:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 258. | Song X, Sturgis EM, Huang Z, Li X, Li C, Wei Q, Li G. Potentially functional variants of p14ARF are associated with HPV-positive oropharyngeal cancer patients and survival after definitive chemoradiotherapy. Carcinogenesis. 2014;35:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 259. | Wu Y, Posner MR, Schumaker LM, Nikitakis N, Goloubeva O, Tan M, Lu C, Iqbal S, Lorch J, Sarlis NJ. Novel biomarker panel predicts prognosis in human papillomavirus-negative oropharyngeal cancer: an analysis of the TAX 324 trial. Cancer. 2012;118:1811-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 260. | Chiosea SI, Grandis JR, Lui VW, Diergaarde B, Maxwell JH, Ferris RL, Kim SW, Luvison A, Miller M, Nikiforova MN. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer. 2013;13:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 261. | Melkane AE, Auperin A, Saulnier P, Lacroix L, Vielh P, Casiraghi O, Msakni I, Drusch F, Temam S. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head Neck. 2014;36:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 262. | Prince A, Aguirre-Ghizo J, Genden E, Posner M, Sikora A. Head and neck squamous cell carcinoma: new translational therapies. Mt Sinai J Med. 2010;77:684-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 263. | Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 264. | Krane JF. Role of cytology in the diagnosis and management of HPV-associated head and neck carcinoma. Acta Cytol. 2013;57:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 265. | Mroz EA, Forastiere AA, Rocco JW. Implications of the oropharyngeal cancer epidemic. J Clin Oncol. 2011;29:4222-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 266. | Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, Kim MS. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012;34:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 267. | McHugh JB. Association of cystic neck metastases and human papillomavirus-positive oropharyngeal squamous cell carcinoma. Arch Pathol Lab Med. 2009;133:1798-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 268. | Thompson LD, Heffner DK. The clinical importance of cystic squamous cell carcinomas in the neck: a study of 136 cases. Cancer. 1998;82:944-956. [PubMed] |
| 269. | Psyrri A, Sasaki C, Vassilakopoulou M, Dimitriadis G, Rampias T. Future directions in research, treatment and prevention of HPV-related squamous cell carcinoma of the head and neck. Head Neck Pathol. 2012;6 Suppl 1:S121-S128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 270. | Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 516] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 271. | Fischer CA, Kampmann M, Zlobec I, Green E, Tornillo L, Lugli A, Wolfensberger M, Terracciano LM. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol. 2010;21:1961-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 272. | Gillison ML. HPV and prognosis for patients with oropharynx cancer. Eur J Cancer. 2009;45 Suppl 1:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 273. | Shaw R, Robinson M. The increasing clinical relevance of human papillomavirus type 16 (HPV-16) infection in oropharyngeal cancer. Br J Oral Maxillofac Surg. 2011;49:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 274. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 4959] [Article Influence: 330.6] [Reference Citation Analysis (0)] |
| 275. | Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 276. | Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhøi BP, Overgaard M, Specht L, Andersen E, Johansen J, Andersen LJ. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6& amp; 7 trial. Radiother Oncol. 2011;100:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 277. | Klozar J, Tachezy R. What are the implications of human papillomavirus status in oropharyngeal tumors for clinical practice? Curr Opin Otolaryngol Head Neck Surg. 2014;22:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 278. | Vu HL, Sikora AG, Fu S, Kao J. HPV-induced oropharyngeal cancer, immune response and response to therapy. Cancer Lett. 2010;288:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 279. | Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, Peet CR, Lorenz LD, Nickel KP, Klingelhutz AJ. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791-4800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 280. | Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, Dikomey E, Kriegs M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 281. | Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1377] [Article Influence: 91.8] [Reference Citation Analysis (1)] |
| 282. | Koutcher L, Sherman E, Fury M, Wolden S, Zhang Z, Mo Q, Stewart L, Schupak K, Gelblum D, Wong R. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 283. | Genden EM. The role for surgical management of HPV-related oropharyngeal carcinoma. Head Neck Pathol. 2012;6 Suppl 1:S98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 284. | Lui VW, Grandis JR. Primary chemotherapy and radiation as a treatment strategy for HPV-positive oropharyngeal cancer. Head Neck Pathol. 2012;6 Suppl 1:S91-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 285. | Mehanna H, Olaleye O, Licitra L. Oropharyngeal cancer is it time to change management according to human papilloma virus status? Curr Opin Otolaryngol Head Neck Surg. 2012;20:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 286. | Garland SM, Smith JS. Human papillomavirus vaccines: current status and future prospects. Drugs. 2010;70:1079-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 287. | Lu B, Kumar A, Castellsagué X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & amp; meta-analysis. BMC Infect Dis. 2011;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 288. | Bhat P, Mattarollo SR, Gosmann C, Frazer IH, Leggatt GR. Regulation of immune responses to HPV infection and during HPV-directed immunotherapy. Immunol Rev. 2011;239:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 289. | Safaeian M, Kemp TJ, Pan DY, Porras C, Rodriguez AC, Schiffman M, Cortes B, Katki H, Wacholder S, Schiller JT. Cross-protective vaccine efficacy of the bivalent HPV vaccine against HPV31 is associated with humoral immune responses: results from the Costa Rica Vaccine Trial. Hum Vaccin Immunother. 2013;9:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 290. | Maeda H, Kubo K, Sugita Y, Miyamoto Y, Komatsu S, Takeuchi S, Umebayashi T, Morikawa S, Kawanishi K, Kameyama Y. DNA vaccine against hamster oral papillomavirus-associated oral cancer. J Int Med Res. 2005;33:647-653. [PubMed] |
| 291. | Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |