Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1967
Peer-review started: January 12, 2024
First decision: January 31, 2024
Revised: February 8, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: April 16, 2024
Ovarian cancer is the most common malignant tumor of the female reproductive system, and the survival rate of patients with relapsed and refractory ovarian cancer is very low.
Here, we report a case of high-grade serous papillary adenocarcinoma of the ovary that was successfully treated with immunotherapy. Radical surgery and adjuvant chemotherapy for the 56-year-old patient were successful; however, her tumor relapsed. Subsequent second-line chemotherapy, targeted agents, and other treatments were ineffective, as the tumor continued to recur and metastasize. Anti-programmed cell death-1 (PD-1) monotherapy (tislelizumab) completely alleviated the tumor, and the multiple metastatic tumors disappeared. To date, the patient has used anti-PD-1 for 32 months, experiencing no disease progression and maintaining good health without additional treatment.
This case suggests that anti-PD-1 immunotherapy may have long-term positive effects on outcomes in some refractory recurrent solid tumors. Further research is needed to identify patients most likely to respond to anti-PD-1 therapy.
Core Tip: When chemotherapy, poly adenosine diphosphate ribose polymerase inhibitors, and other treatments are ineffective for relapsed refractory ovarian cancer, anti-programmed death 1 immunotherapy may be the last resort.
- Citation: Zhou GD, Li Q. Long-term complete response to anti-programmed-death-1 monotherapy in a patient with relapsed and refractory ovarian adenocarcinoma: A case report. World J Clin Cases 2024; 12(11): 1967-1973
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1967.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1967
Ovarian cancer has the highest mortality among all gynecological cancers. Over 200000 new cases of ovarian cancer and 150000 deaths are recorded annually worldwide[1]. As few symptoms occur at the initial stage, nearly 70% of patients are diagnosed at an advanced stage, with few receiving early treatment. Primary epithelial ovarian cancer is the most common pathological type of ovarian cancer, accounting for over 85% of ovarian cancers, with ovarian serous adenocarcinoma accounting for approximately three-quarters of primary ovarian cancers[2]. Following the treatment guidelines of the National Comprehensive Cancer Network (NCCN) for ovarian cancer, the first-line treatment plan for epithelial ovarian cancer is surgery with platinum and paclitaxel-based chemotherapy[3-5]. However, some patients experience tumor recurrence and multiple metastases after various therapies, posing significant challenges to continued treatment. Immunotherapy has been applied to various cancers in recent years. Programmed cell death-1 (PD-1) and its receptor programmed cell death ligand-1 (PD-L1) constitute a signaling pathway involved in tumor immune escape and are common targets for cancer immunotherapy. Anti-PD-1 and anti-PD-L1 antibodies have demonstrated obvious curative efficacy in clinical trials for melanoma, bladder cancer, lung cancer, leukemia, breast cancer, and other malignant tumors[6-8]. They have also proven effective in the adjuvant treatment of ovarian cancer[9-12]. Here, we report our experience with a patient with high-grade serous papillary adenocarcinoma of the ovary, who experienced disease progression and metastasis under multiple therapies. The patient ultimately exhibited a good response to anti-PD-1 monotherapy (tislelizumab) and achieved long-term complete response, with rapid disappearance of the multiple metastatic tumors.
At the age of 46, she visited the hospital after a physical examination revealed a pelvic mass without any complaints of discomfort.
The patient was born in 1968, got married at the age of 23, and, after giving birth to a daughter naturally, used a birth control ring to prevent pregnancy. In 2014, at age 46, she visited the hospital after a physical examination revealed a pelvic mass.
No abnormalities.
The patient had a family history of cancer; her mother had died of lung cancer at 53 years, and her brother had died of bowel cancer at 45 years.
Pelvic examination suggested that both bilateral adnexal areas can be palpated with a mass that extends approximately 6 cm without tenderness.
The patient's carbohydrate antigen 125 (CA125) level was 239.9 U/mL.
Pelvic contrast-enhanced computed tomography (CT) indicated multiple cystic masses (largest 9.0 cm × 8.7 cm × 7.4 cm) with solid papillary nodules on both ovaries, suggesting a high probability of serous cystadenocarcinoma. Chest CT detected no abnormalities. To rule out the possibility of ovarian Krukenberg tumor, we performed a biopsy of a gastroscopy specimen, which indicated mild chronic inflammation of the gastric mucosa. And colonoscopy detected no abnormalities.
The high-grade serous papillary ovarian adenocarcinoma stage IIB (referring to FIGO 2014).
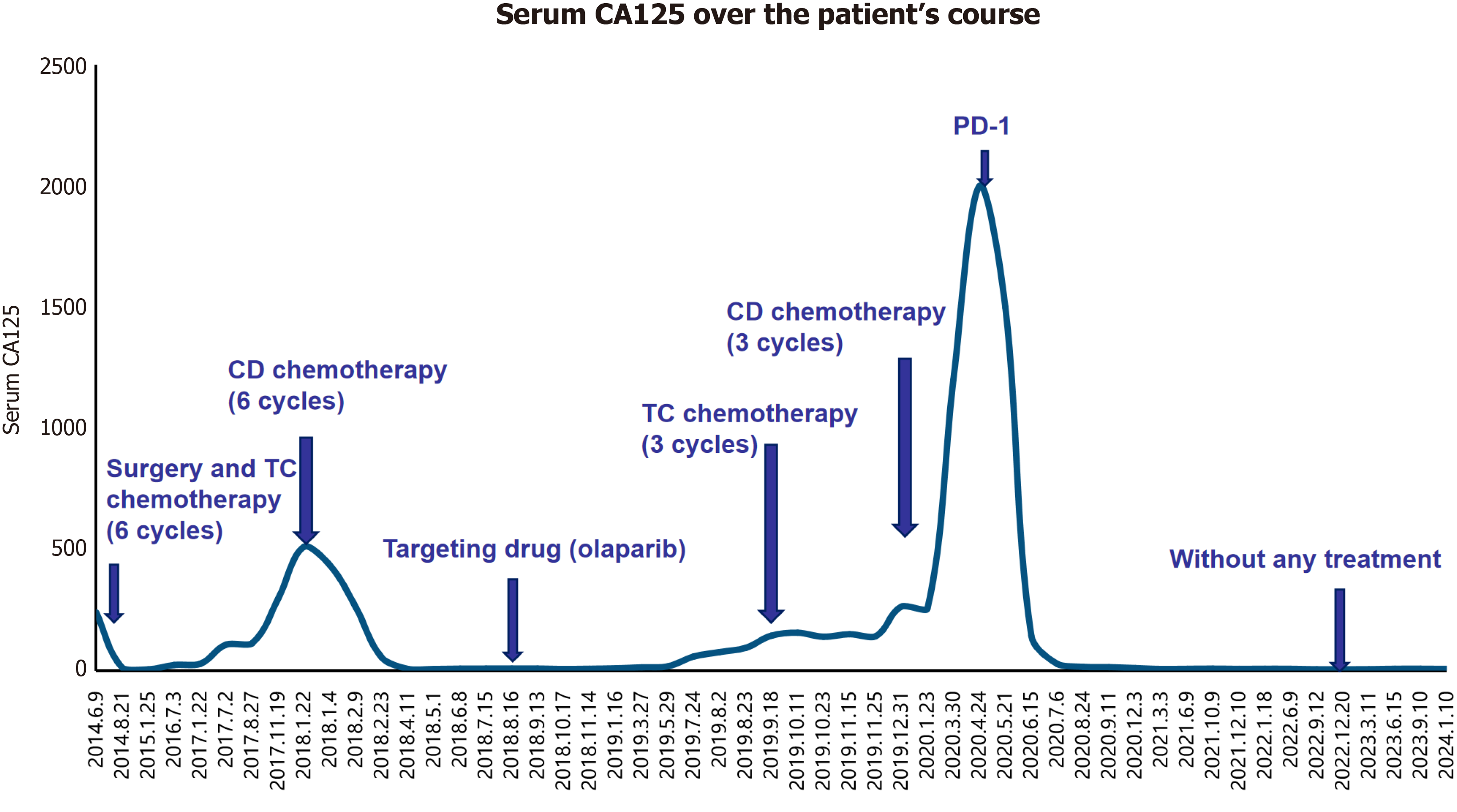
In June 2014, the patient underwent a successful radical resection of ovarian cancer, reaching the R0 level. Pathological examination of the surgical specimen revealed high-grade serous papillary adenocarcinoma of both ovaries, involving the right ovarian fallopian tube. We identified cancer cells in the ascites, but the pelvic lymph nodes, greater omentum, and appendix were negative. The ovarian cancer pathological stage was IIB (referring to FIGO 2014). The patient subsequently received six cycles of first-line chemotherapy with paclitaxel and carboplatin, achieving complete remission. In July 2017, the patient's CA125 began to increase slightly. The first progression-free survival lasted for 2 years. In January 2018, abdominal ultrasonography indicated abdominal lymphadenopathy as well as peripancreatic and hilar solid nodules (maximum size 5.6 cm × 3.1 cm). Following this, she received six courses of second-line chemotherapy with carboplatin and doxorubicin hydrochloride liposome, and her CA125 levels returned to normal. Immediately after six courses of second-line chemotherapy, the patient was treated with oral olaparib as maintenance therapy following the NCCN guideline. A genetic test indicated no BRCA1/2 (Breast Cancer 1/2 mutation) mutations in this patient, the homologous recombination deficiency test is also negative by postoperative genetic testing in August 2018. In July 2019, the patient’s CA125 increased again. The second progression-free survival lasted for 17 months, and she discontinued olaparib in August 2019 (olaparib continued treatment for 1 year from August 2018 to August 2019). Subsequently, she received three courses of carboplatin + paclitaxel from September to November 2019, but her CA125 continued to increase. Therefore, she was switched to three courses of carboplatin + doxorubicin hydrochloride liposome from December 2019 to February 2020. However, her CA125 rose to 2000 U/mL. In March 2020, she presented with multiple nodules in the liver and lungs and multiple enlarged lymph nodes in the neck, mediastinum, and retroperitoneum, indicating multiple metastases.
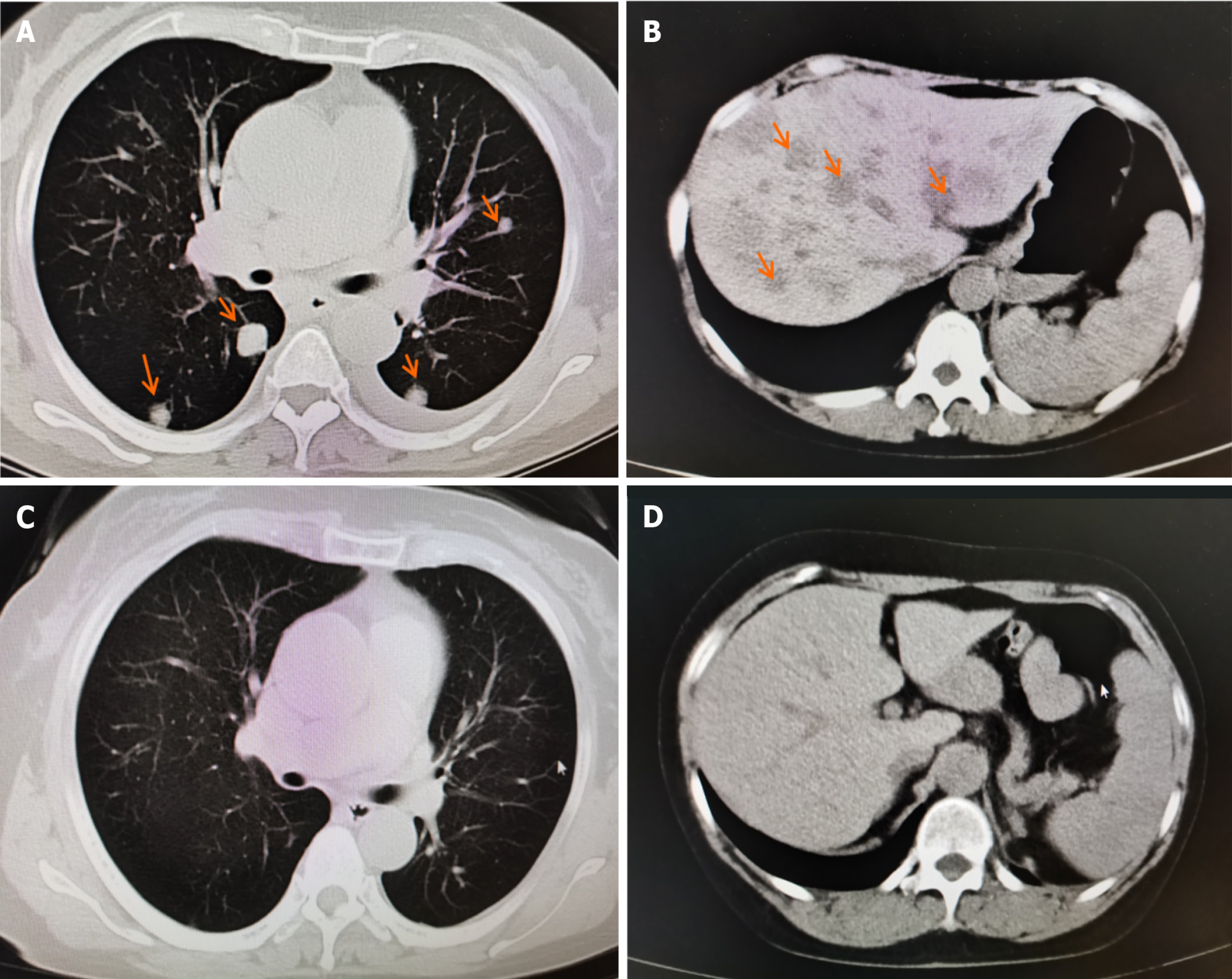
Because surgery, chemotherapy, and targeted drugs (olaparib) failed to control tumor progression, immunotherapy was employed as a last resort. The patient commenced anti-PD-1 monotherapy (tislelizumab, BeiGene Ltd, China, 200 mg intravenous drip every 3–4 wk) in April 2020. The CA125 levels rapidly decreased to normal after three courses of PD1 treatment (Figure 1), the liver and lung metastases disappeared (Figure 2), and the lymph nodes were no longer enlarged, achieving complete remission. She regained good physical condition with no discomfort, and her quality of life returned to normal. Her Eastern Cooperative Oncology Group performance status score was 1. In December 2022, owing to a slight elevation in blood creatinine (reach to 76 umol/L), Tislelizumab monotherapy was discontinued. To date, the third progression-free survival has reached 42 months, with 32 months of PD-1 treatment (April 2020-December 2022) (Treatment process refers to Table 1).
| Date (year, month) | Treatment methods | Duration |
| June 2014 | Operation | / |
| July 2014-January 2015 | TC chemotherapy | 6 months |
| January 2018-June 2018 | CD chemotherapy | 6 months |
| August 2018-August 2019 | Targeting drug (olaparib) | 1 year |
| September 2019-November 2019 | TC chemotherapy | 3 months |
| December 2019-February 2020 | CD chemotherapy | 3 months |
| April 2020-December 2022 | Anti-PD-1 therapy (tislelizumab) | 32 months |
| December 2022-January 2024 | Without any treatment. | 13 months |
The patient is in good health and has normal renal function. Without any complaints of discomfort. She is still being followed up without any treatment.
Ovarian cancer has no obvious symptoms at the early stage; therefore, 80% of patients are diagnosed with advanced disease. The recurrence rate after standard treatment is 80%, and the 5-year survival rate is less than 45%[13,14]. For many patients with refractory ovarian cancer, including ours, effective methods to continue treatment after the failures of surgery, first- and second-line chemotherapy, targeted agents, and other treatment methods are limited. In patients who experience therapy failure repeatedly, the 5-year survival rate is less than 25%[15]. Therefore, immunotherapy represents an important novel treatment option for these patients. However, this procedure is usually expensive. Notably, our patient was treated with tislelizumab, which has a much lower cost than other anti-PD-1 drugs. In China, tislelizumab costs about $200 a dose, translating to approximately $133 per week for the patient. Conversely, nivolumab costs $1250 weekly, and pembrolizumab costs about $1667 weekly. The relatively low price of tislelizumab reduces the financial burden on patients, increasing their likelihood of affording long-term treatment.
Tumors evade the host immune system via different mechanisms, with the immune checkpoint PD-1/PD-L1 playing a crucial role[16]. Anti-PD-1 is often used as an adjunct therapy to chemotherapy and targeted agents. Ovarian cancer cells express higher levels of PD-L1 than control cells, and high PD-L1 expression is an independent risk factor for the prognosis of patients with ovarian cancer[17], suggesting adverse clinical outcomes. Moreover, high expression of PD-L1 in the ascites or circulating monocytes of patients with ovarian cancer is associated with adverse outcomes[18]. Additionally, clinical trial data suggest that anti-PD-1/PD-L1 antibodies benefit patients with ovarian cancer[19]. However, unlike Hodgkin lymphoma, melanoma, and lung cancer, research on the application of anti-PD-1 therapy in treating ovarian cancer is limited. In a clinical trial involving avelumab (anti-PD-L1) in 124 patients with recurrent ovarian cancer, the drug was effective in 9.7% of patients, and 44.4% of patients had stable disease[19]. Compared with traditional PD-1 inhibitors, tislelizumab eliminates the ability to bind to Fc receptors on the surface of macrophages by modifying the Fc segment, resulting in minimal effects of antibody-dependent cellular phagocytosis, antibody-dependent cell-mediated cytotoxicity, and complement-dependent cytotoxicity, avoiding T cell consumption, and exerting stronger anti-tumor effects than other PD-1 inhibitors. Moreover, no serious adverse reactions occur during use (for example the treatment of this case should be stopped immediately if abnormal renal function is detected), and the tolerance is good. More clinical studies and case reports of anti-PD-1/PD-L1 therapy for ovarian cancer are warranted.
To our knowledge, this is the first publicly reported case of advanced, refractory ovarian cancer achieving long-term complete response to anti-PD-1 monotherapy for 32 months. The progression-free survival time has reached 42 months or more. We posit that the heightened sensitivity to anti-PD1 therapy may be related to the patient’s family history of cancer. Our clinical experience suggests that although ovarian clear cell carcinoma is unresponsive to anti-PD1 therapy, ovarian cancer with a family history of cancer, such as Lynch syndrome, is more likely to respond to anti-PD1 therapy. In some cases, anti-PD-1 induces treatment-related adverse events, such as thyroid dysfunction, pneumonia, hip joint pain, pituitary inflammation[20], and lupus erythematosus[21]. Fortunately, our patient developed no adverse events associated with long-term anti-PD-1 therapy for 32 months until a slight rise in serum creatinine prompted cessation. Subsequently, a follow-up was conducted every 3 months. Conventionally, anti-PD1 therapy is used only as an adjunct to chemotherapy or targeted agents when treating ovarian cancer[3,9-12]. However, in our case, complete remission was achieved solely with anti-PD1 monotherapy, which may play a vital role in treating ovarian cancer. Nevertheless, further research is needed to determine which types of patients are more likely to respond to anti-PD1 therapy.
This is the first publicly reported case of advanced, refractory ovarian cancer achieving long-term complete response to anti-PD-1 monotherapy for 32 months. The progression-free survival time has reached 42 months or more. We posit that the heightened sensitivity to anti-PD1 therapy may be related to the patient’s family history of cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thongon N, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Guo X
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19854] [Article Influence: 2206.0] [Reference Citation Analysis (18)] |
| 2. | Kuo HH, Huang CY, Ueng SH, Huang KG, Lee CL, Yen CF. Unexpected epithelial ovarian cancers arising from presumed endometrioma: A 10-year retrospective analysis. Taiwan J Obstet Gynecol. 2017;56:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | NCCN Guidelines for Patients® ovarian cancer, 2023. Available from: www.nccn.org/patientguidelines. [Cited in This Article: ] |
| 4. | Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, Vargas-Aguilar VM. Management of recurrent epithelial ovarian cancer. Gland Surg. 2014;3:198-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 5. | Marth C, Reimer D, Zeimet AG. Front-line therapy of advanced epithelial ovarian cancer: standard treatment. Ann Oncol. 2017;28:viii36-viii39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia; Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia; Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med. 2016;374:998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Yang X, Yin R, Xu L. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;379:e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3742] [Cited by in F6Publishing: 3573] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2457] [Cited by in F6Publishing: 2515] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 10. | Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 11. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5947] [Article Influence: 495.6] [Reference Citation Analysis (0)] |
| 12. | Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33:4015-4022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 717] [Cited by in F6Publishing: 818] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 13. | Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 552] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 14. | Korkmaz T, Seber S, Basaran G. Review of the current role of targeted therapies as maintenance therapies in first and second line treatment of epithelial ovarian cancer; In the light of completed trials. Crit Rev Oncol Hematol. 2016;98:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Antoni Basta, Mariusz Bidziński, Andrzej Bieńkiewicz, Paweł Blecharz, Lubomir Bodnar, Robert Jach, Paweł Knapp, Zbigniew Kojs, Jan Kotarski, Janina Markowska, Marcin Misiek, Jacek Sznurkowski, Łukasz Wicherek, Włodzimierz Sawicki, Agnieszka Timorek, Jan Bahyrycz, Radosław Mądry. Recommendation of the Polish Society of Oncological Gynaecology on the diagnosis and treatment of epithelial ovarian cancer. Oncol Clini Pract. 2015;11:233-243. [Cited in This Article: ] |
| 16. | Fan CA, Reader J, Roque DM. Review of Immune Therapies Targeting Ovarian Cancer. Curr Treat Options Oncol. 2018;19:74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. |
Huang W, Chen B.
The expression of PD-L1 and PD-L2 in ovarian carcinoma and its prognostic significance. |
| 18. | Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, George AJ, Ghaem-Maghami S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81:17-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 344] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 20. | Johnson C, Jazaeri AA. Diagnosis and Management of Immune Checkpoint Inhibitor-related Toxicities in Ovarian Cancer: A Series of Case Vignettes. Clin Ther. 2018;40:389-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ceccarelli F, Mancuso S, Lucchetti R, Conti F. Systemic lupus erythematosus onset in patient receiving anti-PD1 treatment with pembrolizumab: a case report. Rheumatology (Oxford). 2021;60:e39-e40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |