Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1960
Peer-review started: December 17, 2023
First decision: January 10, 2024
Revised: February 25, 2024
Accepted: March 18, 2024
Article in press: March 18, 2024
Published online: April 16, 2024
Syphilis is an infectious disease caused by Treponema pallidum that can invade the central nervous system, causing encephalitis. Few cases of anti-N-methyl-D-aspartate receptor autoimmune encephalitis (AE) secondary to neurosyphilis have been reported. We report a neurosyphilis patient with anti-γ-aminobutyric acid-B receptor (GABABR) AE.
A young man in his 30s who presented with acute epileptic status was admitted to a local hospital. He was diagnosed with neurosyphilis, according to serum and cerebrospinal fluid (CSF) tests for syphilis. After 14 d of antiepileptic treatment and anti-Treponema pallidum therapy with penicillin, epilepsy was controlled but serious cognitive impairment, behavioral, and serious psychiatric symptoms were observed. He was then transferred to our hospital. The Mini-Mental State Examination (MMSE) crude test results showed only 2 points. Cranial magnetic resonance imaging revealed significant cerebral atrophy and multiple fluid-attenuated inversion recovery high signals in the white matter surrounding both lateral ventricles, left amygdala and bilateral thalami. Anti-GABABR antibodies were discovered in CSF (1:3.2) and serum (1:100). The patient was diagnosed with neurosyphilis complicated by anti-GABABR AE, and received methylprednisolone and penicillin. Following treatment, his mental symptoms were alleviated. Cognitive impairment was significantly improved, with a MMSE of 8 points. Serum anti-GABABR antibody titer decreased to 1:32. The patient received methylprednisolone and penicillin after discharge. Three months later, the patient’s condition was stable, but the serum anti-GABABR antibody titer was 1:100.
This patient with neurosyphilis combined with anti-GABABR encephalitis benefited from immunotherapy.
Core Tip: In this report, we investigated the complex interplay between neurosyphilis and autoimmune encephalitis (AE), specifically anti-γ-aminobutyric acid-B receptor AE. Our findings shed light on the intricate connections between syphilis-related neurological complications and autoimmune responses, highlighting the potential significance of targeted immunotherapies in managing such cases. This investigation contributes valuable insights into the understanding and treatment of neurosyphilis, emphasizing the relevance of considering autoimmune mechanisms in its pathogenesis.
- Citation: Fang YX, Zhou XM, Zheng D, Liu GH, Gao PB, Huang XZ, Chen ZC, Zhang H, Chen L, Hu YF. Neurosyphilis complicated by anti-γ-aminobutyric acid-B receptor encephalitis: A case report. World J Clin Cases 2024; 12(11): 1960-1966
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1960.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1960
Neurosyphilis is one of the systemic complications of syphilis caused by Treponema pallidum. The prognosis of neurosyphilis varies considerably based on specific populations and disease stages[1-3]. Widespread inflammation has been observed in the brain following neurosyphilis infection. There have been reported cases of syphilis combined with autoimmune encephalitis (AE), such as antibodies against N-methyl-D-aspartate receptor (anti-NMDAR) AE[4-7], or demyelinating diseases, such as antibodies against aquaporin-4 (anti-AQP4) neuromyelitis optica spectrum disorder (NMOSD) in which immunotherapy, including methylprednisolone or immunoglobulin treatment, has shown benefits in the prognosis of neurosyphilis patients[8-10]. In this report, we present a case of neurosyphilis complicated by anti-γ-aminobutyric acid-B receptor (GABABR) AE.
A young man in his 30 s was admitted to our hospital with complaints of recurrent seizures accompanied by behavioral and psychiatric changes, such as not recognizing family members, inability to communicate, and excited for more than 17 d.
The patient suddenly developed epileptic seizures and was confused for intermittent periods 17 d previously. He was taken to a local hospital and was considered to have an “epileptic status”, and was given symptomatic treatment such as “propofol, midazolam, and sodium valproate”. Neurosyphilis was considered as the patient’s serum (1:16) and cerebrospinal fluid (CSF, 1:4) TRUST titer test was positive. After 14 d of penicillin treatment, the patient developed psychiatric and behavior disorders and was unable to communicate effectively. He was treated with “valproate 0.4 g bid and olanzapine 5 mg bid”. His symptoms did not significantly improve, and he was referred to our hospital.
He denied a history of infection, diarrhea, fever, or other previous medical history.
He had no history of drinking, smoking or drug use. His parents were both in good health.
Neurological examination revealed slow response, difficulty in language expression, personality change, attention and short-term memory impairments, unstable mood, and impulsive behavior. The patient had no other pathological signs.
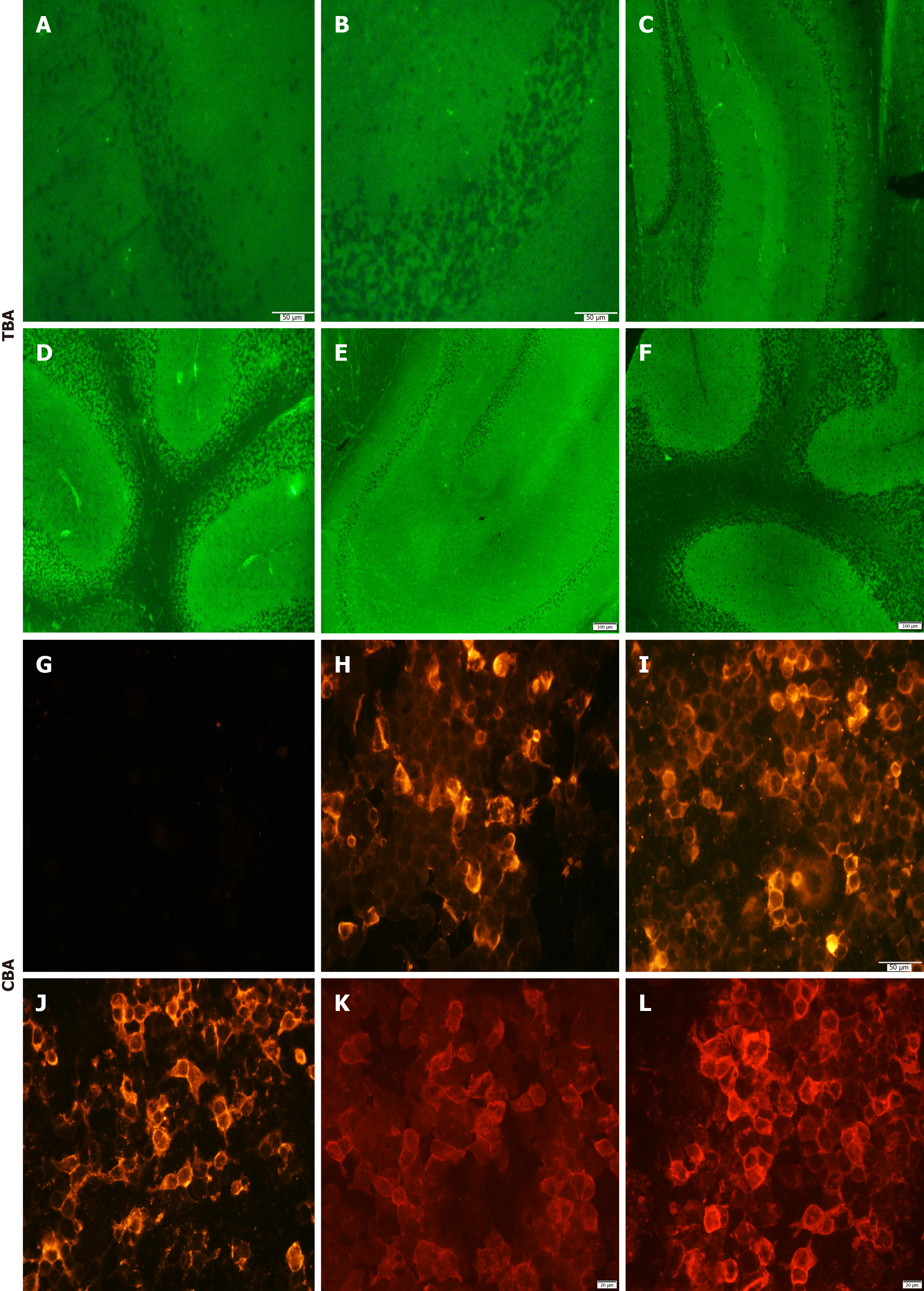
There were no significant abnormalities in routine blood tests, including biochemistry and coagulation. Tumor markers were in the normal range. The test results for other infections [herpes simplex viruses (HSV), varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, etc.] were negative, and syphilis titers were re-examined on admission, and the TRUST titer was 1:4 in serum and 1:1 in CSF. Pleocytosis (white blood cells of 12 × 106/L) and increased protein concentration (0.50 g/L) were found in CSF. Oligoclonal protein electrophoresis was positive in CSF. In addition, the patient’s tissue-based assay (TBA) results were compared with TBA-negative examples (Figure 1A and B). TBA of the CSF sample revealed positive neuronal immunoreaction (Figure 1C-F), and a cell-based assay (CBA) for known autoantigens of AE in the serum and CSF samples were screened and compared with GABABR-negative examples (Figure 1G). GABABR autoantibody was identified in serum (1:100) and CSF (1:3.2) (Figure 1H and I). The test results for other autoantibodies (NMDAR, AQP4, Hu, Yo, Ri, etc.) were negative. After 2 weeks, serum-GABABR was positive (1:32) (Figure 1J). After 3 months, CSF-GABABR was positive (1:10) (Figure 1K), and serum-GABABR was positive (1:100) (Figure 1L). A Mini-Mental State Examination (MMSE) score of 2/30 was confirmed.
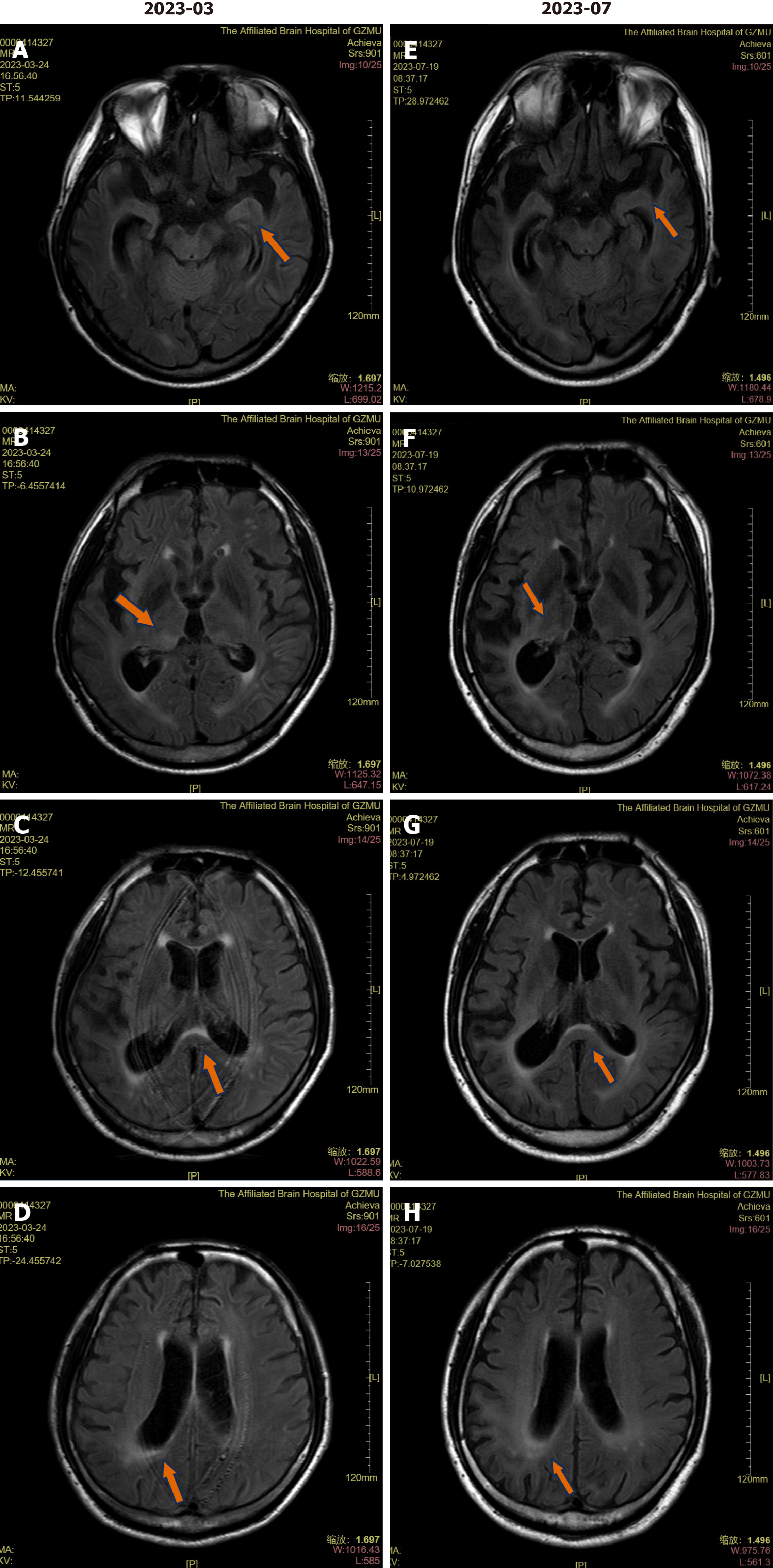
Brain magnetic resonance imaging (MRI) showed significant cerebral atrophy and multiple fluid-attenuated inversion recovery (FLAIR) high signals in the white matter surrounding both lateral ventricles, left amygdala and bilateral thalami (Figure 2). The patient’s chest computed tomography (CT) scan showed no significant abnormalities.
Based on the clinical examinations and results of TRUST titer and anti-GABABR antibodies in CSF, the patient was diagnosed with neurosyphilis complicated by anti-GABABR AE.
According to the patient’s weight, he received intravenous methylprednisolone 1 g QD for 3 d, 0.5 g QD for 3 d, 0.25 g QD for 3 d, and 0.125 g QD for 3 d. Twelve d after methylprednisolone therapy, his psychiatric and behavioral disorders disappeared and his cognitive impairment improved, with the MMSE score increasing from 2/30 to 8/30. The serum TRUST titer decreased to 1:4, and serum GABABR antibody titer decreased to 1:32 (Figure 1J). The venereal disease research laboratory test (VDRL) and GABABR antibody detection were not tested due to a lack of CSF samples.
After discharge, the patient was given intramuscular injections of 2.4 million units of penicillin once a week for three weeks. He continued to take methylprednisolone orally at a dose of 60 mg/d, and the dosage was then gradually tapered by one-third every week until the drug was completely withdrawn. His condition remained stable without further aggravation. Three months later, repeat serum TRUST titer was still 1:4, CSF VDRL was negative, but Treponema pallidum hemagglutination assay was still positive, and serum and CSF GABABR antibody titer were 1:100 and 1:10, respectively (Figure 1L and K), the MMSE score was 8/30, and cranial MRI showed that the FLAIR high signals in the white matter surrounding both lateral ventricles, left amygdala and bilateral thalami, and brain atrophy was the same as before (Figure 2). Intravenous methylprednisolone shock therapy was given again for 9 d (0.5 g QD for 3 d, 0.25 g QD for 3 d, 0.125 g QD for 3 d), and the MMSE score increased to 11 points.
In the present report, we described a case of neurosyphilis complicated by anti-GABABR AE, which is to our knowledge, the first reported case. GABABR is widely expressed in the brain, including the limbic system (such as amygdala), thalamus, and cerebellum. It is involved in the activity of dopaminergic and other monoaminergic neurons by binding to the inhibitory neurotransmitter GABA[11]. Most of the patients with anti-GABABR AE are middle-aged and elderly men, who usually have acute or subacute onset. The main clinical manifestations include epilepsy, mental disorders, and memory loss. Approximately half of the patients with anti-GABABR AE have abnormal cranial MRI of the medial temporal lobe. One-third of patients present with small-cell lung cancer[12]. However, this patient’s tumor markers were within the normal range, and chest CT showed no significant abnormalities. There was no evidence of tumor.
Our case had typical acute onset manifestations. His electroencephalogram showed a diffuse slow wave, cranial MRI showed significant cerebral atrophy and multiple FLAIR high signals in the white matter surrounding both lateral ventricles, left amygdala and bilateral thalami. Why did the acute course of disease cause significant brain atrophy? We think it is unlikely that such significant brain atrophy occurred as a short-term consequence of brain damage and the brain atrophy in this patient might reflect long-standing progression. Patients with asymptomatic neurosyphilis can show brain atrophy, despite showing no symptoms. Approximately 75% of patients with neurosyphilis have been reported to show normal or nonspecific brain atrophy on cranial MRI, which may reflect a quiescent and prolonged course of syphilis, whereas parenchymal lesions in the temporal lobe and thalami can be seen in both early and late stages of neurosyphilis[13,14]. Patients with neurosyphilis have varying clinical and neuroimaging features, including cerebral infarction or hemorrhage, atrophy, demyelination, arteritis, encephalitis, and hippocampal sclerosis[15]. Therefore, this patient’s brain atrophy may be related to years of latent syphilis infection, reflecting the long-term progression of the disease. The hyperintense lesions in the medial temporal region and white matter (such as splenium of corpus callosum etc.) on the patient’s cranial MRI were associated with the development of seizures, cognitive deficits, and psychobehavioral abnormalities, and all of these imaging abnormalities, especially lesions of the limbic system, are typical of neurosyphilis and anti-GABABR encephalitis.
Infection is a risk factor for AE, and AE has been reported following HSV, severe acute respiratory syndrome coronavirus 2 infection etc.[16,17]. Syphilis, also known as “the great imitator”, and neurosyphilis are both widely known to share clinical features with many diseases. Recently, AE triggered by syphilis has been increasingly recognized[4,18,19]. The cause of secondary AE following syphilis may be due to syphilis directly injuring brain tissue, releasing neuronal proteins capable of inducing autoantibody production and central nervous system damage. There have been several reported cases of anti-NMDAR AE complicated by syphilis[5,6], in which immunotherapy has improved the outcome of patients and is recommended in complicated cases. In the present case, penicillin and antiepileptic treatment only improved seizures but not cognitive impairment and mental abnormalities. Following methylprednisolone shock therapy, the patient’s psychiatric and behavioral disorders disappeared and his cognitive impairment improved, accompanied by a decrease in the anti-GABABR titer (1:100 to 1:32) and an increase in the MMSE score from 2 to 8. Although the patient continued to take methylprednisolone orally for one month, three months later the patient’s cognitive impairment did not continue to improve, and the MMSE remained at about 8/30 points. Thus, we reviewed the patient’s CSF syphilis titer and GABABR titer and found that the VDRL was negative, but the anti-GABABR antibody titer in serum and CSF had increased again. After another round of methylprednisolone treatment, the patient’s MMSE score increased to 11/30. These data indicate that the cognitive damage caused by syphilis may be partially worsened by anti-GABABR AE, and immunotherapy intervention is necessary.
Therefore, for infectious diseases of the central nervous system, if symptoms do not significantly improve after targeted anti-infective treatment, antibody testing is of crucial clinical significance. It plays an important role in disease warning, guiding diagnosis and treatment, and evaluating prognosis. It is also worth noting that AE cannot be ruled out based solely on a negative antibody test result. The most commonly used methods for antibody testing in clinical settings are CBA and TBA, both of which are indirect immunofluorescence techniques[20,21], CBA can only detect about 30 known antibodies, leaving many unknown antibodies undiscovered. Therefore, when CBA is negative but the patient’s clinical manifestations meet the criteria for AE, TBA results should be considered. TBA-positivity indicates the presence of antigen-antibody reactions but does not specify the type of antibody (it could be a known or unknown antibody), and it can provide information on the different brain regions and subcellular localization of the antibodies. Our team previously conducted a retrospective analysis of 81 patients diagnosed with neurosyphilis and found that TBA positive staining was significantly correlated with head MRI abnormalities (P < 0.001 for parenchymal abnormalities and P = 0.013 for white matter lesions). The cognitive prognosis of TBA-positive neurosyphilis patients was significantly worse than that of TBA-negative patients (P < 0.001)[22].
Our patient with syphilis complicated by anti-GABABR AE was timely diagnosed and immunotherapy in addition to anti-syphilis treatment was beneficial in this patient.
Syphilis in combination with AE (e.g. anti-NMDAR AE) or demyelinating disease (e.g. anti-AQP4 NMOSD) has been previously reported. However, to date, cases of neurosyphilis combined with anti-GABABR AE have rarely been reported. If the characteristics of neurosyphilis combined with anti-GABABR AE are defined, we will be able to identify, diagnose, and treat these patients earlier.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Osawa I, Japan S-Editor: Zhang L L-Editor: Webster JR P-Editor: Guo X
| 1. | Zhou P, Gu X, Lu H, Guan Z, Qian Y. Re-evaluation of serological criteria for early syphilis treatment efficacy: progression to neurosyphilis despite therapy. Sex Transm Infect. 2012;88:342-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Walter T, Lebouche B, Miailhes P, Cotte L, Roure C, Schlienger I, Trepo C. Symptomatic relapse of neurologic syphilis after benzathine penicillin G therapy for primary or secondary syphilis in HIV-infected patients. Clin Infect Dis. 2006;43:787-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Marra CM, Sahi SK, Tantalo LC, Ho EL, Dunaway SB, Jones T, Hawn TR. Toll-like receptor polymorphisms are associated with increased neurosyphilis risk. Sex Transm Dis. 2014;41:440-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Beiruti K, Abu Awad A, Keigler G, Ryder CH, Shahien R. Atypical development of neurosyphilis mimicking limbic encephalitis. Int J STD AIDS. 2019;30:194-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Li XY, Shi ZH, Guan YL, Ji Y. Anti-N-methyl-D-aspartate-receptor antibody encephalitis combined with syphilis: A case report. World J Clin Cases. 2020;8:2603-2609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (4)] |
| 6. | Qin K, Wu W, Huang Y, Xu D, Zhang L, Zheng B, Jiang M, Kou C, Gao J, Li W, Zhang J, Wang S, Luan Y, Yan C, Zheng X. Anti-N-methyl-D-aspartate receptor(NMDAR) antibody encephalitis presents in atypical types and coexists with neuromyelitis optica spectrum disorder or neurosyphilis. BMC Neurol. 2017;17:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Chen HQ, Zhang Y, Wang SB, Song YN, Bai MS, Liu KD, Zhu MQ. Concurrent aquaporin-4-positive NMOSD and neurosyphilis: A case report. Mult Scler Relat Disord. 2019;34:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Liao H, Zhang Y, Yue W. Case Report: A Case Report of Neurosyphilis Mimicking Limbic Encephalitis. Front Neurol. 2022;13:862175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 9. | Tsukita K, Shimotake A, Nakatani M, Takahashi Y, Ikeda A, Takahashi R. [A case of neurosyphilis presenting with limbic encephalitis]. Rinsho Shinkeigaku. 2017;57:37-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Wilcox RA, Burrow J, Slee M, Craig J, Thyagarajan D. Neuromyelitis optica (Devic's disease) in a patient with syphilis. Mult Scler. 2008;14:268-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Heaney CF, Kinney JW. Role of GABA(B) receptors in learning and memory and neurological disorders. Neurosci Biobehav Rev. 2016;63:1-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de Felipe A, Saiz A, Dalmau J, Graus F. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81:1500-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 13. | Jum'ah A, Aboul Nour H, Alkhoujah M, Zoghoul S, Eltous L, Miller D. Neurosyphilis in disguise. Neuroradiology. 2022;64:433-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Czarnowska-Cubała M, Wiglusz MS, Cubała WJ, Jakuszkowiak-Wojten K, Landowski J, Krysta K. MR findings in neurosyphilis--a literature review with a focus on a practical approach to neuroimaging. Psychiatr Danub. 2013;25 Suppl 2:S153-S157. [PubMed] [Cited in This Article: ] |
| 15. | Shang XJ, He CF, Tang B, Chang XL, Ci C, Sang H. Neuroimaging Features, Follow-Up Analyses, and Comparisons Between Asymptomatic and Symptomatic Neurosyphilis. Dermatol Ther (Heidelb). 2020;10:273-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Patil VA, Kulkarni SD, Udwadia-Hegde A, Sayed RJ, Garg M. Anti-N-methyl-D-aspartate receptor encephalitis during relapse of herpes simplex encephalitis in a young boy: A brief review of literature. Neurol India. 2017;65:393-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune Encephalitis After SARS-CoV-2 Infection: Case Frequency, Findings, and Outcomes. Neurology. 2021;97:e2262-e2268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Serrano-Cardenas KM, Sánchez-Rodriguez A, Pozueta A, Pelayo AL, Riancho J. Mesial encephalitis: an uncommon presentation of neurosyphilis: a case report and review of the literature. Neurol Sci. 2018;39:173-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Mizoguchi T, Hara M, Nakajima H. Neurosyphilis presenting as autoimmune limbic encephalitis: A case report and literature review. Medicine (Baltimore). 2022;101:e30062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Molina RD, Conzatti LP, da Silva APB, Goi LDS, da Costa BK, Machado DC, Sato DK. Detection of autoantibodies in central nervous system inflammatory disorders: Clinical application of cell-based assays. Mult Scler Relat Disord. 2020;38:101858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Masi G, Spagni G, Campetella L, Monte G, Sabatelli E, Evoli A, Papi C, Iorio R. Assessing the role of a tissue-based assay in the diagnostic algorithm of autoimmune encephalitis. J Neuroimmunol. 2021;356:577601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Fang Y, Wu H, Liu G, Li Z, Wang D, Ning Y, Pan S, Hu Y. Secondary immunoreaction in patients with neurosyphilis and its relevance to clinical outcomes. Front Neurol. 2023;14:1201452. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |