Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1940
Peer-review started: November 2, 2023
First decision: December 7, 2023
Revised: January 2, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: April 16, 2024
Direct carotid cavernous fistulas (CCFs) are typically the result of a severe traumatic brain injury. High-flow arteriovenous shunts secondary to rupture of an intracavernous aneurysm, resulting in direct CCFs, are rare. The use of a pipeline embolization device in conjunction with coils and Onyx glue for treatment of direct high-flow CCF resulting from ruptured cavernous carotid artery aneurysm in a clinical setting is not well documented.
A 58-year-old woman presented to our department with symptoms of blepharoptosis and intracranial bruits for 1 wk. During physical examination, there was right eye exophthalmos and ocular motor palsy. The rest of the neurological examination was clear. Notably, the patient had no history of head injury. The patient was treated with a pipeline embolization device in the ipsilateral internal carotid artery across the fistula. Coils and Onyx were placed through the femoral venous route, followed by placement of the pipeline embolization device with assistance from a balloon-coiling technique. No intraoperative or perioperative complications occurred. Preoperative symptoms of bulbar hyperemia and bruits subsided immediately after the operation.
Pipeline embolization device in conjunction with coiling and Onyx may be a safe and effective approach for direct CCFs.
Core Tip: A patient with direct carotid cavernous fistula was treated with a pipeline embolization device in the ipsilateral internal carotid artery across the fistula. Additionally, coils and Onyx were placed through the femoral venous route, followed by placement of the pipeline embolization device with assistance from a balloon-coiling technique. No intraoperative or perioperative complications occurred. Preoperative symptoms of bulbar hyperemia and bruits subsided immediately after the operation.
- Citation: Ouyang G, Zheng KL, Luo K, Qiao M, Zhu Y, Pan DR. Endovascular treatment of direct carotid cavernous fistula resulting from rupture of intracavernous carotid aneurysm: A case report. World J Clin Cases 2024; 12(11): 1940-1946
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1940.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1940
Direct carotid cavernous fistulas (CCFs) are abnormal vessel connections between the cavernous sinus and the internal carotid artery (ICA)[1]. Direct CCF is typically the result of a severe traumatic brain injury. Laceration of the cavernous segment of the ICA wall or avulsion of the cavernous ICA wall by trauma may lead to direct CCF. Spontaneous carotid cavernous sinus fistula is rare. High-flow arteriovenous shunts secondary to a ruptured intracavernous aneurysm resulting in a direct CCF are also rare. Indirect CCF is also known as cavernous sinus dural arteriovenous fistula and compression of the carotid artery may spontaneously resolve it. However, direct CCF and most indirect CCFs require surgical treatment.
Endovascular intervention of these lesions is usually the main treatment. In the past, CCF was treated with a balloon that occluded the ICA at the expense of sacrificing it[2]. In recent decades, there has been a significant development of endovascular techniques in the treatment of these challenging lesions, such as CCF. Treatments include a detachable balloon that obliterates the fistula, cavernous sinus filled with coils and liquid embolic agent to eliminate the shunt, and covered stent placement to obliterate direct CCF. Detachable balloons, coils, liquid embolic agents, and covered stents are now commonly used in endovascular therapy[3,4]. The development of flow-diversion devices has provided further options for treatment.
For small-caliber fistulas, coil embolization can be used alone, but larger shunts tend to require utilizing adjunct devices such as nondetachable balloons or stents to ensure the patency of the ICA. A pipeline endovascular device (PED) can be used for this purpose.
Cavernous sinus surrounding structures such as cranial nerves III, IV, V, and VI can be affected on the ipsilateral side of the cavernous sinus congestion. Clinical presentation can also include abducens palsy, exophthalmos, epistaxis, chemosis, and bruits. The ideal outcome of CCF treatment is complete occlusion of the fistula while maintaining the patency of the carotid artery and preserving the integrity of the cranial nerves. A model treatment of CCF secondary to rupture of intracavernous aneurysm is absolute occlusion of the shunt, to avoid recurrent rupture of the aneurysm and to treat the paralysis caused by the aneurysmal pressure against the cerebral nerves.
Blepharoptosis with intracranial murmur for 1 wk.
A 58-year-old woman presented with ptosis of the left eyelid accompanied by pulsatile tinnitus. According to the patient's self-report, these symptoms suddenly appeared 1 wk previously and gradually worsened, resulting in limited vision and hearing discomfort.
The patient was in good health before. She had no history of infectious disease, food or drug allergy, or surgery. She also denied having a history of head trauma or other related diseases, as well as diseases in other systems.
The patient’s parents are both alive.
During physical examination, there was right eye exophthalmos and ocular motor palsy. The remaining neurological examination was normal.
No obvious abnormality was found.
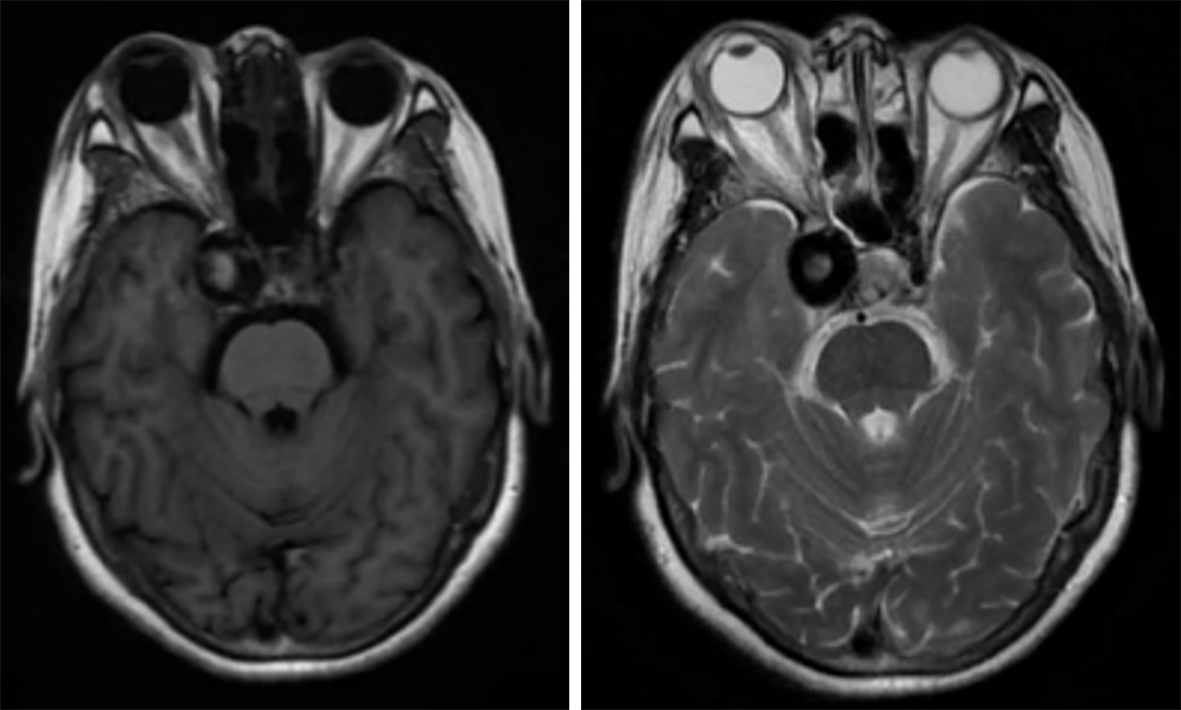
Magnetic resonance imaging revealed a right-sided aneurysm (Figure 1), with tortuous and increased vascular shadow in the posterior space of the right eye.
The final diagnosis was direct CCF.
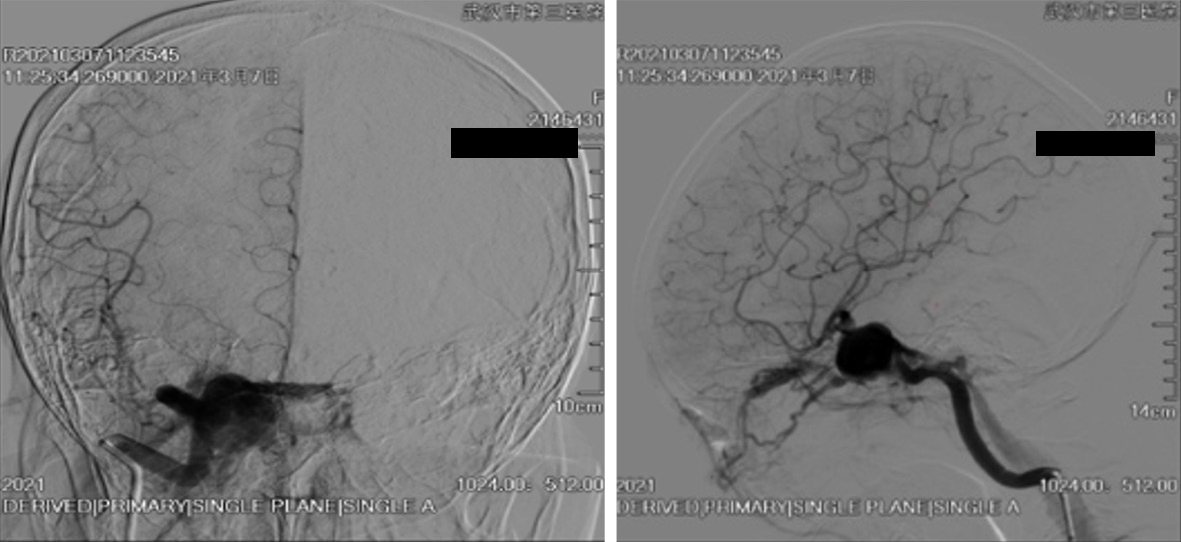
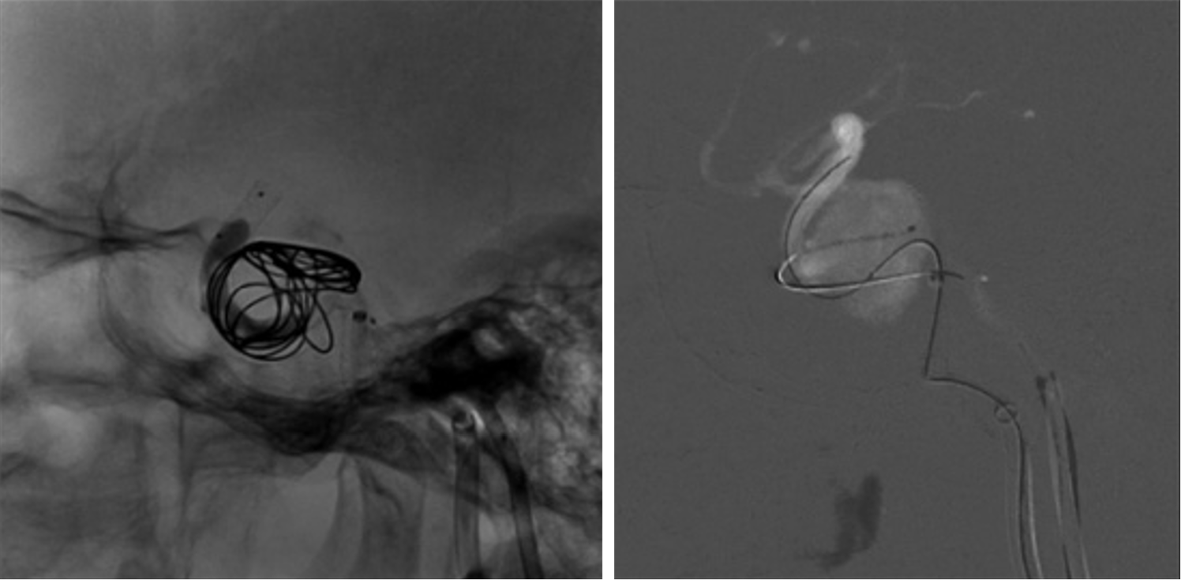
We decided to perform endovascular treatment of the CCF and the aneurysm using a combined transarterial and transvenous approach. Preoperatively, the patient was pretreated with 100 mg aspirin and 75 mg clopidogrel for several days. Clopidogrel was substituted with ticagrelor after the thromboelastography test showed a low platelet inhibition rate. Intraoperatively, right ICA angiography demonstrated a ruptured giant cavernous carotid aneurysm with a fistulous outflow via the ipsilateral subpetrosal sinus and the ipsilateral superior ophthalmic vein (Figure 2). A PED was deployed in the ICA across the aneurysmal neck via a transfemoral approach and the procedures were performed under general anesthesia. Under Traxcess-14 micro-guidewire guidance, an Eechelon-10 microcatheter was inserted via the femoral vein, across the subpetrosal sinus, then through the right-side cavernous sinus, and into the aneurysmal sac. We withdrew the micro-guidewire and filled the aneurysmal sac with several coils through the Eechelon-10 microcatheter. Onyx glue was injected through the microcatheter slowly and intermittently under the protection of the Scepter balloon. Immediate postoperative angiography showed normal patency of the parent ICA and a marked reduction in arteriovenous shunting (Figure 3). As for the residual shunting, the surgeon thought that it would disappear with thrombus formation (Figure 4). Postoperatively, dual antiplatelet therapy with ticagrelor (90 mg/d) and aspirin (100 mg/d) was continued. The day after the operation, aspirin was discontinued due to hematuria. The patient received single antiplatelet therapy with ticagrelor 90 mg twice daily, which was maintained for 3 mo.
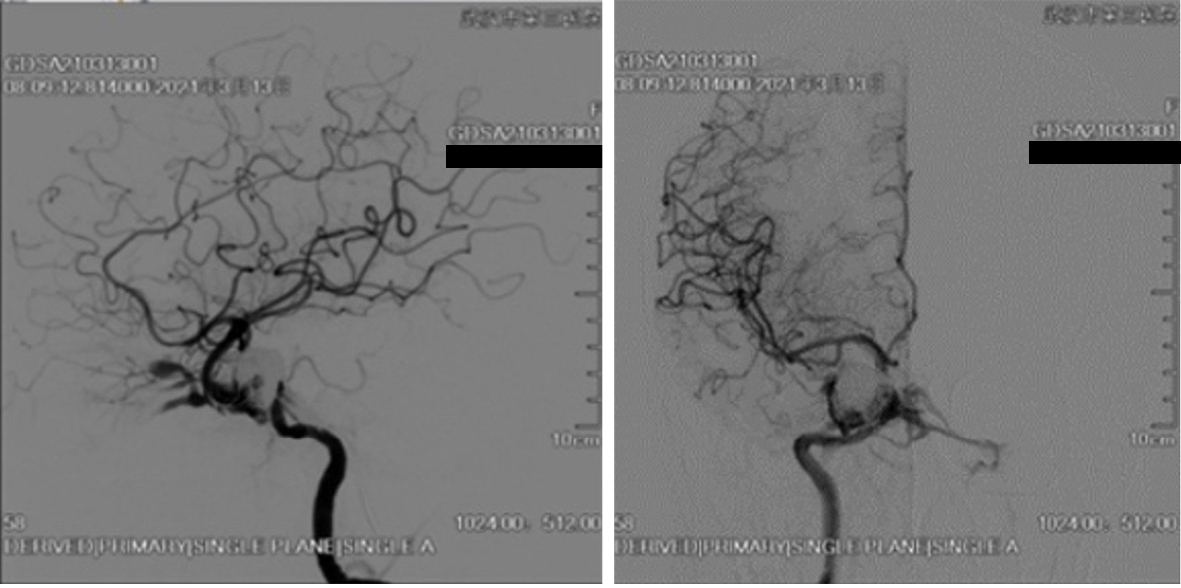
The patient’s symptoms of right eye hyperemia and bruits immediately disappeared after surgery. Digital subtraction angiography (DSA) demonstrated only minimal residual fistulous flow, consistent with the patient’s gradual recovery from thrombosis. After 3 mo of follow-up, the patient had no neurological defects, and the right eye proptosis and chemosis disappeared. DSA confirmed total obliteration of the fistula with stable occlusion of the aneurysm, concurrent with complete resolution of the symptoms.
PED is a brief but unique versatile device composed of cobalt–chromium and platinum that provides 30%–35% metal surface area coverage and is self-expandable[5]. Many cerebrovascular pathologies that are considered untreatable by conventional techniques have been treated after the introduction of flow-diverting stent technology. As for anatomically complex aneurysms, PED is a superior choice to surgical clipping and endovascular coiling.
Although PED was approved by the United States FDA only for wide-neck ICA aneurysms, it has gained wide acceptance as a treatment approach for unruptured intracranial aneurysms, including small aneurysms and other types of aneurysms, with techniques and indications for PED use still evolving.
Distal aneurysms are those located beyond the circle of Willis. PEDs can be used to treat distal aneurysms located at or beyond the A2 segment of the anterior cerebral artery and the P2 segment of the posterior cerebral artery. The M2 segment of the middle cerebral artery may incur a high rate of occlusion and ischemic events, but PED is a feasible option for aneurysms in the pericallosal artery and those that are not manageable with simple coiling. The main challenges of PED treatment for distal aneurysms lie in the fact that its release system is harder than that of conventional stents, and PED release requires a larger diameter catheter[6]. The treatment of wide-necked bifurcation aneurysm via PED is easier than by unassisted or assisted coiling. The complication rate of ischemic events and occlusion after bifurcation aneurysms treated by flow diversion devices (FDD) is high, so this approach is only suggested when the surgical and other endovascular approaches are unfeasible. The main concern of PED therapy for bifurcated aneurysms is perioperative or late thromboembolic events of PED-covered branches and perforating vessels[7].
FDD can also be applied to small aneurysms with lower ischemic complication rates[8]. PED is also suitable for complex ruptured small aneurysms that cannot be treated by other methods, especially for blood-blister-like aneurysms[9]. For aneurysm recurrence after endovascular treatment or previously clipped aneurysm, flow diversion is also a reasonable option. For recurrent aneurysms with previously stent-assisted embolization, compared with those without stent-assisted embolization, FDD treatment has a poor outcome and an increased incidence of adverse events. The presence of a previous stent will result in the PED apposition to the vessel wall and incomplete opening, triggering ischemic events. Nine anterior communicating aneurysms postsurgical clipping have been reported, with a complete occlusion rate of 83% and no perioperative complications[10].
It is still controversial whether PEDs for large or giant aneurysms should be combined with coiling. It is generally believed that PED in conjunction with coiling would be more effective. However, recent studies have shown that PED combined with coiling treatment of intracranial aneurysm reduces the probability of aneurysm rupture and has an obstructive effect on aneurysms, causing them to shrink or disappear.
Compared with conventional endovascular therapy, PED cannot effectively treat lesions immediately and may delay the healing process. Also, the stent may prolapse without support[11]. The use of coils in conjunction with pipelines can resolve this problem. Coils in the aneurysmal sac can act as a scaffold to avoid the stent protruding into the aneurysm. Coils can reduce the incidence of postoperative aneurysm rupture and hemorrhage through the embologenic and mechanical support effect of coiling, and can also reduce the incidence of aneurysmal rupture and recurrence caused by PED shortening or postoperative displacement.
Coiling may cause the PED to open badly or cause incomplete stent apposition. Studies have shown that the incidence of early ischemic events after PED combined with coil embolization is significantly higher than that after PED alone. Lin et al reported that, among 104 patients with complex aneurysms, 29 were treated with PED and coils, and 75 were treated with PED alone. It was concluded that complete aneurysmal occlusion was achieved in a higher proportion of cases treated with the pipeline plus coils compared with pipeline only and reduced the need for retreatment. There was no significant difference in periprocedural and delayed complications between the two groups[12].
Although PEDs are mainly used for treatment of particular internal carotid aneurysms, recent reports describe their off-label use for CCF treatment. Concerning CCFs, Baranoski et al[13] described a case of traumatic CCF that was treated in a multisession approach, initially deploying two PEDs across the fistulous site to provide double coverage, followed by transvenous coiling of the inferior ophthalmic vein and cavernous sinus. DSA demonstrated complete obliteration of the fistula with no residual shunting. For the low-flow types A and B CCFs, one single PED may be sufficient due to a low-pressure gradient. PED can induce thrombosis by reducing flow, providing a permanent closure by endothelization of the CCF[14].
The sole use of multiple overlapping PEDs has also been described in the treatment of CCF. Without embolic agents into the cavernous sinus, multiple PEDs can also efficiently reduce high-flow fistula and avoid cranial nerve injury during a dense coil mass within the cavernous sinus[15,16].
The current literature shows that using PED for treatment of CCF is likely to be safe and effective[17]. First, PED can reduce the use of coil or embolization materials, thereby reducing the mass effect in the aneurysmal sac or cavernous sinus, as wells as cranial nerve complications due to the mass effect. Second, PED as a barrier can reduce the risk of embolization material such as coils and liquid penetrating into the ICA, increase the safety of liquid embolization agent occlusion of the fistula through venous approach, and improve the cure rate. Compared with balloon occlusion, PED can reduce the risk of ischemia rate. Third, PEDs can promote the formation of thrombosis at the fistula by reducing the flow. Many reviews suggest that flow diversion may be a useful tool in the treatment of CCF.
Most CCF patients with residual shunting on angiography after PED deployment were found to be occluded at later follow-up. This shows that PEDs can affect flow rate and promote thrombosis.
The role of PED is based on the two mechanisms of blood flow guidance and promotion of the cervical endoderm to promote aneurysmal healing. Compared with the traditional stent, a PED can better reshape the parent artery at the neck of the aneurysm[14,18]. Aneurysmal diameter and morphology, parent artery diameter ratio, cervical ratio, collateral artery, and gender are risk factors for postoperative occlusion of aneurysms. PEDs have been shown to have good safety and efficacy for the treatment of complex internal carotid aneurysms. Follow-up appointments confirmed that 86.8% and 95.2% of aneurysms had complete occlusion at 1–5 years postoperatively.
The main drawback of PED treatment for CCF is the time that it takes for the fistula to become occluded, which may delay symptomatic improvement. In addition, PED is expensive and requires dual antiplatelet therapy, but this method is simple and reduces the risk of cranial nerve damage[19,20].
The complications of flow diversion mainly include ischemic stroke and cerebral hemorrhage, which are the mass effects of giant aneurysms. The occurrence of ischemic stroke is mainly caused by the stent adhering to the vessel wall or poor opening of the stent. Multiple PEDs or stenting of the parent artery may lead to inadequate stent opening. Also, parent artery stenosis or side branch artery occlusion may cause ischemic stroke. Mechanical compression or detachment of the thrombosis can occlude the branch vessel. Cerebral hemorrhage includes delayed aneurysmal rupture and delayed lobar cerebral hemorrhage. Delayed aneurysmal rupture is caused by continuous blood flow in the aneurysm after FDD treatment and continuous enlargement of thrombosis leading to aneurysmal rupture. Delayed cerebral hemorrhage is associated with vascular wall damage during FDD treatment, especially with dual antiplatelet therapy. In addition, cerebral hemorrhage may be associated with increased postoperative blood flow[21]. There is a risk of further postope
We believe that the use of PED in conjunction with coils and/or liquid embolic agents for the treatment of CCF secondary to a rupture of an intracavernous aneurysm may be a safe and effective strategy. PED can protect the parent vessel during transvenous coiling or liquid embolization infusion. For the aneurysm with residual shunting, PED-induced alterations in flow contribute to thrombosis. Dual antiplatelet therapy may lead to bleeding complications and its dose should be adjusted when needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ennab RM, Jordan S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006;27:185-189. [PubMed] [Cited in This Article: ] |
| 2. | Scialfa G, Valsecchi F, Scotti G. Treatment of vascular lesions with balloon catheters. AJNR Am J Neuroradiol. 1983;4:395-398. [PubMed] [Cited in This Article: ] |
| 3. | Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol. 2010;12:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | He XH, Li WT, Peng WJ, Lu JP, Liu Q, Zhao R. Endovascular treatment of posttraumatic carotid-cavernous fistulas and pseudoaneurysms with covered stents. J Neuroimaging. 2014;24:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Fiorella D, Woo HH, Albuquerque FC, Nelson PK. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-20; discussion 1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Limbucci N, Leone G, Renieri L, Nappini S, Cagnazzo F, Laiso A, Muto M, Mangiafico S. Expanding Indications for Flow Diverters: Distal Aneurysms, Bifurcation Aneurysms, Small Aneurysms, Previously Coiled Aneurysms and Clipped Aneurysms, and Carotid Cavernous Fistulas. Neurosurgery. 2020;86:S85-S94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Michelozzi C, Darcourt J, Guenego A, Januel AC, Tall P, Gawlitza M, Bonneville F, Cognard C. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: complications, aneurysm sac occlusion, reabsorption, recurrence, and jailed branch modification at follow-up. J Neurosurg. 2018;131:1751-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Nossek E, Zumofen D, Nelson E, Raz E, Potts MB, Desousa KG, Tanweer O, Shapiro M, Becske T, Riina HA. Use of Pipeline Embolization Devices for treatment of a direct carotid-cavernous fistula. Acta Neurochir (Wien). 2015;157:1125-9; discussion 1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Linfante I, Mayich M, Sonig A, Fujimoto J, Siddiqui A, Dabus G. Flow diversion with Pipeline Embolic Device as treatment of subarachnoid hemorrhage secondary to blister aneurysms: dual-center experience and review of the literature. J Neurointerv Surg. 2017;9:29-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Jia L, Wang J, Zhang L, Zhang Y, You W, Yang X, Lv M. Evaluating the Tubridge™ flow diverter for large cavernous carotid artery aneurysms. Chin Neurosurg J. 2020;6:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Crowley RW, Abla AA, Ducruet AF, McDougall CG, Albuquerque FC. Novel application of a balloon-anchoring technique for the realignment of a prolapsed pipeline embolization device: a technical report. J Neurointerv Surg. 2014;6:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lin N, Brouillard AM, Krishna C, Mokin M, Natarajan SK, Sonig A, Snyder KV, Levy EI, Siddiqui AH. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery. 2015;76:142-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Baranoski JF, Ducruet AF, Przbylowski CJ, Almefty RO, Ding D, Catapano JS, Brigeman S, Fredrickson VL, Cavalcanti DD, Albuquerque FC. Flow diverters as a scaffold for treating direct carotid cavernous fistulas. J Neurointerv Surg. 2019;11:1129-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Lin LM, Iyer RR, Bender MT, Monarch T, Colby GP, Huang J, Tamargo RJ, Coon AL. Rescue Treatment with Pipeline Embolization for Postsurgical Clipping Recurrences of Anterior Communicating Artery Region Aneurysms. Interv Neurol. 2017;6:135-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Ogilvy CS, Motiei-Langroudi R, Ghorbani M, Griessenauer CJ, Alturki AY, Thomas AJ. Flow Diverters as Useful Adjunct to Traditional Endovascular Techniques in Treatment of Direct Carotid-Cavernous Fistulas. World Neurosurg. 2017;105:812-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Amuluru K, Al-Mufti F, Gandhi CD, Prestigiacomo CJ, Singh IP. Direct carotid-cavernous fistula: A complication of, and treatment with, flow diversion. Interv Neuroradiol. 2016;22:569-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Stamatopoulos T, Anagnostou E, Plakas S, Papachristou K, Lagos P, Samelis A, Derakhshani S, Mitsos A. Treatment of carotid cavernous sinus fistulas with flow diverters. A case report and systematic review. Interv Neuroradiol. 2022;28:70-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, Fiorella D, Jabbour P, Levy E, McDougall C, Siddiqui A, Szikora I, Woo H, Albuquerque F, Bozorgchami H, Dashti SR, Delgado Almandoz JE, Kelly ME, Turner R 4th, Woodward BK, Brinjikji W, Lanzino G, Lylyk P. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015;36:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 400] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Sumdani H, Aguilar-Salinas P, Avila MJ, El-Ghanem M, Dumont TM. Carotid Cavernous Fistula Treatment via Flow Diversion: A Systematic Review of the Literature. World Neurosurg. 2021;149:e369-e377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Vakharia K, Munich SA, Waqas M, Levy EI, Siddiqui AH. Treatment of Anterior Circulation Aneurysms in the Internal Carotid Artery With Flow Diverters. Neurosurgery. 2020;86:S55-S63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Brunozzi D, Shakur SF, Hussein AE, Charbel FT, Alaraj A. Middle cerebral artery flow velocity increases more in patients with delayed intraparenchymal hemorrhage after Pipeline. J Neurointerv Surg. 2018;10:249-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |