Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1936
Peer-review started: September 26, 2023
First decision: December 5, 2023
Revised: December 30, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: April 16, 2024
Processing time: 198 Days and 4.9 Hours
Li-Fraumeni syndrome (LFS) is a rare autosomal dominant cancer-predisposing syndrome, which can manifest as a polymorphic spectrum of malignancies. LFS is associated with an early onset in life, with the majority of cases occurring prior to the age of 46. Notwithstanding the infrequency of primary cardiac tumors, it behooves clinicians to remain vigilant in considering the differential diagnosis of such tumors in LFS patients who present with a cardiac mass. This is due to the markedly elevated risk for malignancy in this particular population, far surpass
Herein, we present a case of a 30-year-old female with LFS who was found to have a tricuspid valve leaflet mass.
This case exemplifies valuable learning points in the diagnostic approach for this exceptionally rare patient population.
Core Tip: Li-Fraumeni syndrome is a rare autosomal dominant cancer-predisposing syndrome, which can manifest as a polymorphic spectrum of malignancies.
- Citation: Huffaker T, Pak S, Asif A, Otchere P. Tricuspid mass-curious case of Li-Fraumeni syndrome: A case report. World J Clin Cases 2024; 12(11): 1936-1939
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1936.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1936
Li-Fraumeni syndrome (LFS) is a rare familial tumor predisposition syndrome caused by an autosomal dominant mutation in a p53 tumor suppressor gene on chromosome 17[1]. LFS manifests itself in various ways across both the genotypic and phenotypic spectra. Despite the broad range of phenotypic expressions, this syndrome is associated with a specific set of cancers, which includes soft-tissue sarcoma, osteosarcoma, premenopausal breast cancer, brain tumor, adrenocortical carcinoma, leukemia, or bronchoalveolar lung cancer. LFS is also characterized by a predisposition to develop various types of cancers at a relatively young age. Nearly 50% of affected men and women develop an LFS-associated malignancy by 46 for men and by 31 for women[2]. According to the Chompret criteria, a proband is diagnosed with LFS if they meet at least one of the following conditions: (1) Diagnosis of an LFS-associated malignancy before the age of 46; (2) Having one or more first or second-degree relatives who were diagnosed with an LFS-associated malignancy before the age of 56; and (3) Having one or more first or second-degree relatives with multiple tumors, regardless of the age of onset[1]. These criteria are essential for identifying individuals who may have an inherited predisposition to LFS. Diagnosis and genetic testing based on these criteria can help determine the risk of cancer and allow for appropriate medical monitoring and preventive measures for affected individuals and their families.
The incidence of LFS has been reported to range from a mere 0.05% to 0.2% on a global scale, classifying it as an exceedingly rare condition[3]. Consequently, this rarity has contributed to a dearth of comprehensive knowledge concerning LFS within the medical community. Furthermore, specific percentage data regarding the risk of primary cardiac tumor among LFS patients compared to the general population remains conspicuously absent. Nevertheless, it is imperative to emphasize that a well-established association exists between LFS and a significantly heightened overall risk of cancer. In light of this, it stands to reason that individuals afflicted with LFS face a notably elevated susceptibility to various forms of cancer, encompassing the peril of primary cardiac tumor, when juxtaposed against their counterparts lacking this hereditary syndrome. Herein, we present a case of a 30-year-old female with LFS who was found to have a tricuspid valve leaflet mass. This case serves as an illustration of important lessons in the diagnostic approach for an exceptionally rare group of patients, offering valuable insights for learning.
A 30-year-old female was referred to our cardio-oncology clinic due to a cardiac mass in the right ventricle, which was detected during an annual surveillance echocardiography for two atrial septal defects.
Her medical history was notable for LFS and a personal history of various cancers, including breast cancer, ovarian cancer, as well as soft tissue and bone sarcoma.
At the time of evaluation, the patient reported no recent episodes of chest pain, palpitations, exertional dyspnea, orthopnea, or peripheral edema. The physical exam also showed no evidence of a murmur, arrhythmia, jugular venous distention, crackles in the lung field, or leg swelling.
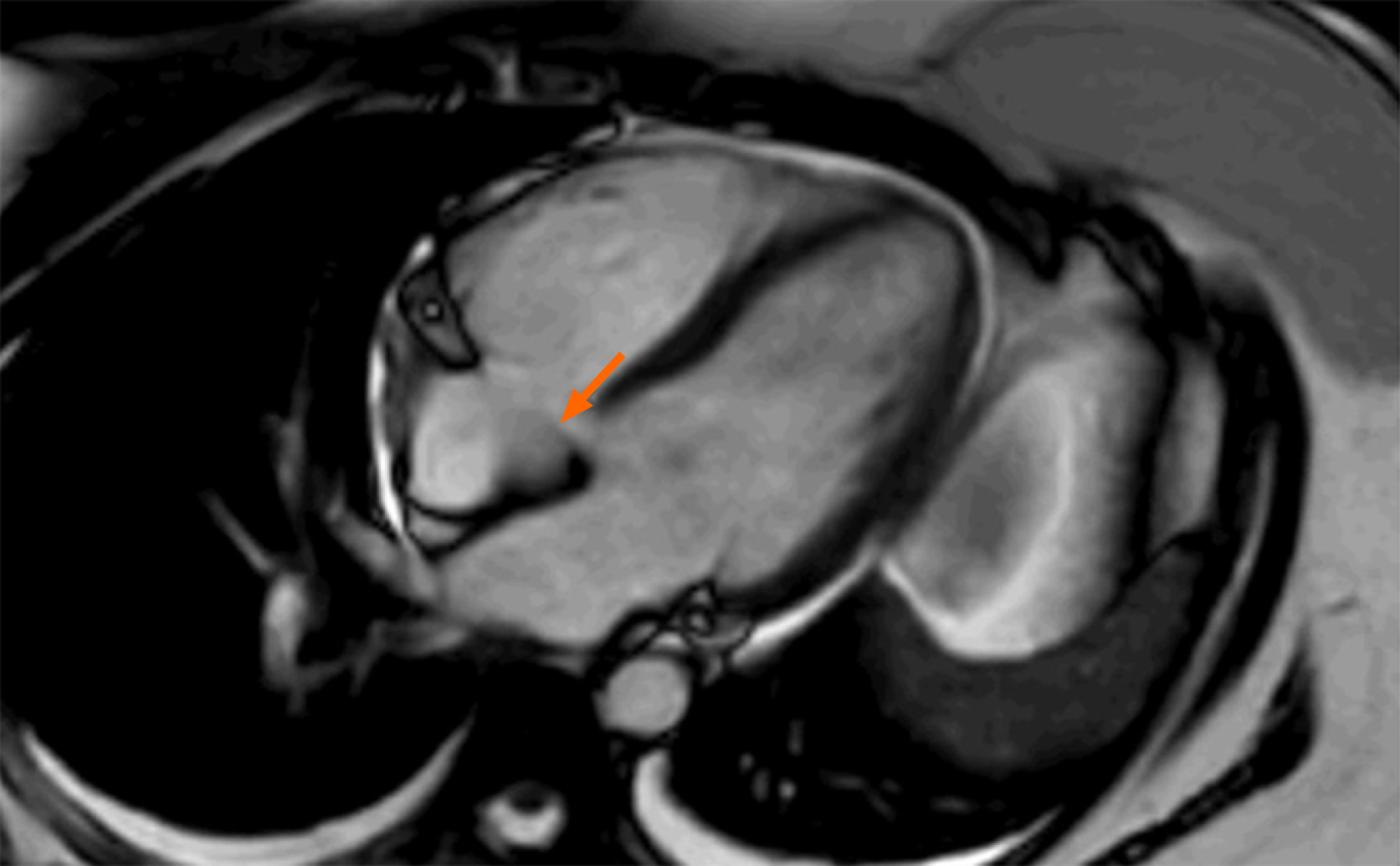
The review of the transthoracic echocardiography (TTE) revealed a 1 cm × 1 cm mass arising from the tricuspid valve (Video 1). This mass was not present on the TTE conducted a year prior to this TTE, indicating it is a new finding. Considering the patient’s complex medical history, it was decided to pursue a comprehensive approach involving transesophageal echocardiogram (TEE) and cardiovascular magnetic resonance imaging (MRI) to further evaluate her cardiac mass. TEE revealed an echogenic mass attached to the anterior tricuspid valve leaflet, measuring 1.22 cm × 0.735 cm. Cardiac MRI visualized a highly mobile mass in the anterior leaflet of the tricuspid valve (Figure 1).
In light of her previous cancer history, the finding of a new cardiac mass raised concerns for possible primary cardiac tumor or metastasis. We had a multi-disciplinary approach including cardio-oncology, multimodal cardiac imaging specialty, cardiothoracic surgery, interventional cardiology, and pediatric and adult oncology. Together, with the patient, the consensus was to surgically remove the mass.
Intracardiac thrombus.
Subsequently, the patient underwent cardiothoracic surgery. Her biopsy showed a 2 cm mass arising from the posterior leaflet of the tricuspid valve with a thin stalk. This mass was excised at the stalk. The specimen was identified as a large, organized thrombus with no evidence of malignancy. Her atrial septal defects were also successfully closed in during the surgery. Her postoperative recovery was uneventful.
The patient experienced no complications at the 1-month follow-up after surgery.
Cardiac tumors are uncommon conditions, traditionally diagnosed postmortem, but with the progress in imaging techniques, they are now more frequently encountered in clinical practice. Due to its widespread availability and excellent temporal-spatial resolution, TTE stands as the primary method of investigation for cardiac masses, ensuring accurate assessment of hemodynamic impacts[4]. While some cardiac masses can be easily identified through TTE alone, others may necessitate the integration of more advanced technologies, such as cardiovascular MRI, and TEE for better visualization. Cardiovascular MRI offers robust tissue characterization with high-contrast resolution, enabling precise differentiation between benign and malignant cardiac lesions. Its first pass perfusion enables detection of regions of relative hyper-perfusion, which is typically seen in malignant lesions. Cardiovascular MRI offers a robust tissue characterization with high-contrast resolution, playing a pivotal role in distinguishing between benign and malignant cardiac tumors. With its first-pass perfusion capability, cardiovascular MRI makes it easy to spot regions with relative hyper-perfusion—a major indicator commonly linked to malignant lesions[5].
In the case we present, the cardiac mass, which was found to be a thrombus, was shown to have an enhancement on the cardiovascular MRI. However, it should not have exhibited enhancement, as it lacks a vasculature. False enhancement is known to be exceedingly rare with cardiovascular MRI, although the specific rate has not yet been reported due to its scarcity. This case highlights the importance of not solely relying on cardiovascular MRI for the identification of cardiac masses, particularly when suspicion for malignancy is high.
Also, the case exemplifies an efficient approach for managing a cardiac mass in patients at high risk of developing primary cardiac tumors or cardiac metastases. Patients are at high risk for cardiac thrombosis when they exhibit the following characteristics: the presence of cancer in another part of the body, personal history of cancer in the past, LFS, Carney complex, asbestos exposure, or family history of primary cardiac tumor[6-8].
If the cardiovascular MRI showed features more consistent with a thrombus, the preferred approach would have been a catheter-based mechanical thrombectomy. This typically utilizes an angiographic catheter with a suction mechanism. Thrombolytic agents are not recommended in these cases due to the risk of thrombus fragments traveling from the right side of the heart to the lungs, potentially leading to a pulmonary embolism.
The lessons drawn from this case can serve as a stepping stone towards enhancing the overall management of cardiac masses and improving patient prognosis. Furthermore, as medical knowledge evolves, further research in this area has the potential to lead to even more effective strategies for managing cardiac masses in high-risk patient populations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO, Netherlands S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Rocca V, Blandino G, D'Antona L, Iuliano R, Di Agostino S. Li-Fraumeni Syndrome: Mutation of TP53 Is a Biomarker of Hereditary Predisposition to Tumor: New Insights and Advances in the Treatment. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Kumamoto T, Yamazaki F, Nakano Y, Tamura C, Tashiro S, Hattori H, Nakagawara A, Tsunematsu Y. Medical guidelines for Li-Fraumeni syndrome 2019, version 1.1. Int J Clin Oncol. 2021;26:2161-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | de Andrade KC, Mirabello L, Stewart DR, Karlins E, Koster R, Wang M, Gapstur SM, Gaudet MM, Freedman ND, Landi MT, Lemonnier N, Hainaut P, Savage SA, Achatz MI. Higher-than-expected population prevalence of potentially pathogenic germline TP53 variants in individuals unselected for cancer history. Hum Mutat. 2017;38:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | D'Angelo EC, Paolisso P, Vitale G, Foà A, Bergamaschi L, Magnani I, Saturi G, Rinaldi A, Toniolo S, Renzulli M, Attinà D, Lovato L, Lima GM, Bonfiglioli R, Fanti S, Leone O, Saponara M, Pantaleo MA, Rucci P, Di Marco L, Pacini D, Pizzi C, Galiè N. Diagnostic Accuracy of Cardiac Computed Tomography and 18-F Fluorodeoxyglucose Positron Emission Tomography in Cardiac Masses. JACC Cardiovasc Imaging. 2020;13:2400-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Chan AT, Plodkowski AJ, Pun SC, Lakhman Y, Halpenny DF, Kim J, Goldburg SR, Matasar MJ, Moskowitz CS, Gupta D, Steingart R, Weinsaft JW. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Oliveira GH, Al-Kindi SG, Hoimes C, Park SJ. Characteristics and Survival of Malignant Cardiac Tumors: A 40-Year Analysis of >500 Patients. Circulation. 2015;132:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors--diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Bussani R, Castrichini M, Restivo L, Fabris E, Porcari A, Ferro F, Pivetta A, Korcova R, Cappelletto C, Manca P, Nuzzi V, Bessi R, Pagura L, Massa L, Sinagra G. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr Cardiol Rep. 2020;22:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |