Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.148
Peer-review started: September 5, 2023
First decision: November 22, 2023
Revised: November 30, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 6, 2024
Elevated levels of cardiac troponin and abnormal electrocardiogram changes are the primary basis for clinical diagnosis of acute coronary syndrome (ACS). Troponin levels in ACS patients can often be more than 50 times the upper reference limit. Some patients with subarachnoid hemorrhage (SAH) also show electrocardiogram abnormalities, myocardial damage, and elevated cardiac biomarkers. Unlike ACS patients, patients with SAH only have a slight increase in troponin, and the use of anticoagulants or antiplatelet drugs is prohibited. Because of the opposite treatment modalities, it is essential for clinicians to distinguish between SAH and ACS.
A 56-year-old female patient was admitted to the emergency department at night with a sudden onset of severe back pain. The final diagnosis was intraspinal hematoma in the thoracic spine. We performed an emergency thoracic spinal canal hematoma evacuation procedure with the assistance of a microscope. Intraoperatively, diffuse hematoma formation was found in the T7-T10 spinal canal, and no obvious spinal vascular malformation changes were observed. Postoperative head and spinal magnetic resonance imaging (MRI) showed a small amount of SAH in the skull, no obvious abnormalities in the cervical and thoracic spinal canals, and no abnormal signals in the lumbar spinal canal. Thoracoabdominal aorta computed tomography angiography showed no vascular malfor
Extremely elevated troponin levels (more than 50 times the normal range) are not unique to coronary artery disease. SAH can also result in extremely high troponin levels, and antiplatelet drugs are contraindicated in such cases. Emergency MRI can help in the early differential diagnosis, as a misdiagnosis of ACS can lead to catastrophic neurological damage in patients with spontaneous spinal SAH.
Core Tip: Elevated cardiac troponin levels and abnormal electrocardiographic changes are the primary basis for the clinical diagnosis of acute coronary syndrome (ACS). Some patients with subarachnoid hemorrhage (SAH) have similar features. Unlike patients with ACS, patients with SAH have only mildly elevated troponin, and antiplatelet agents are contraindicated. We report for the first time a patient with spontaneous spinal SAH of unknown etiology, who was misdiagnosed as having ACS because of severe back pain, very high troponin levels, and abnormal electrocardiographic changes, and who developed catastrophic neurological damage after treatment with oral antiplatelet agents.
- Citation: Lin JM, Yuan XJ, Li G, Gan XR, Xu WH. Subarachnoid hemorrhage misdiagnosed as acute coronary syndrome leading to catastrophic neurologic injury: A case report. World J Clin Cases 2024; 12(1): 148-156
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/148.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.148
Acute coronary syndrome (ACS) is a severe cardiovascular disease characterized by ischemic symptoms in the cardiac supply region due to coronary artery disease[1]. ACS includes ST elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina. Cardiac troponin levels and electrocardiographic findings are the primary basis for the clinical diagnosis of STEMI and NSTEMI, and antiplatelet agents are key in pretreatment[2]. Although substantial progress has been made in diagnosing and treating ACS, ischemic heart disease remains a leading cause of death worldwide[3]. ACS is considered to be a medical emergency, and treatment outcomes largely depend on rapid diagnosis and treatment approach.
Similar to the clinical presentation of ACS in patients, some patients with intraspinal hematomas present with electrocardiogram abnormalities, myocardial damage, and elevated cardiac biomarkers. Intraspinal hematomas are uncommon and can be divided into three types: Intramedullary, subdural, and epidural hematomas. Among them, spinal subdural hematomas are the rarest, accounting for only 4% of intraspinal hematomas[4,5]. Subarachnoid hemorrhage (SAH) of the spinal cord is the primary cause of spinal subdural hematomas. Spontaneous spinal SAH is usually associated with spinal vascular malformations, coagulation disorders, and the use of anticoagulants or antiplatelet drugs[6]. It is considered a neurological emergency that requires early diagnosis and active treatment to salvage neurological function. Anticoagulants or antiplatelet drugs are contraindicated in patients with spinal SAH.
Troponin levels in patients with ACS are usually more than 50 times the upper reference limit[7]. Unlike patients with ACS, patients with SAH have only slightly elevated troponin levels, which differentiates the two. Herein, we report, for the first time, a case of catastrophic neurological injury in a patient with spontaneous spinal SAH of unknown etiology who was misdiagnosed with ACS and treated with oral antiplatelet agents because of severe thoracic and back pain, extremely high troponin levels, and abnormal electrocardiographic changes. In this case, it is clear that very high troponin levels may not be suitable to clearly distinguish ACS from SAH.
A 56-year-old female patient was admitted to the emergency department at night with a sudden onset of severe back pain.
Four hours prior, the patient engaged in heavy lifting, with no chest tightness or chest pain.
The patient had no history of medication use and no history of hypertension, tumors, or blood diseases.
The patient had no remarkable personal and family history.
Motor system examination showed that the strength of the lower limb was Medical Research Council Scale (MRC) grade 5/5, and the Visual Analog Scale (VAS) score was 6.
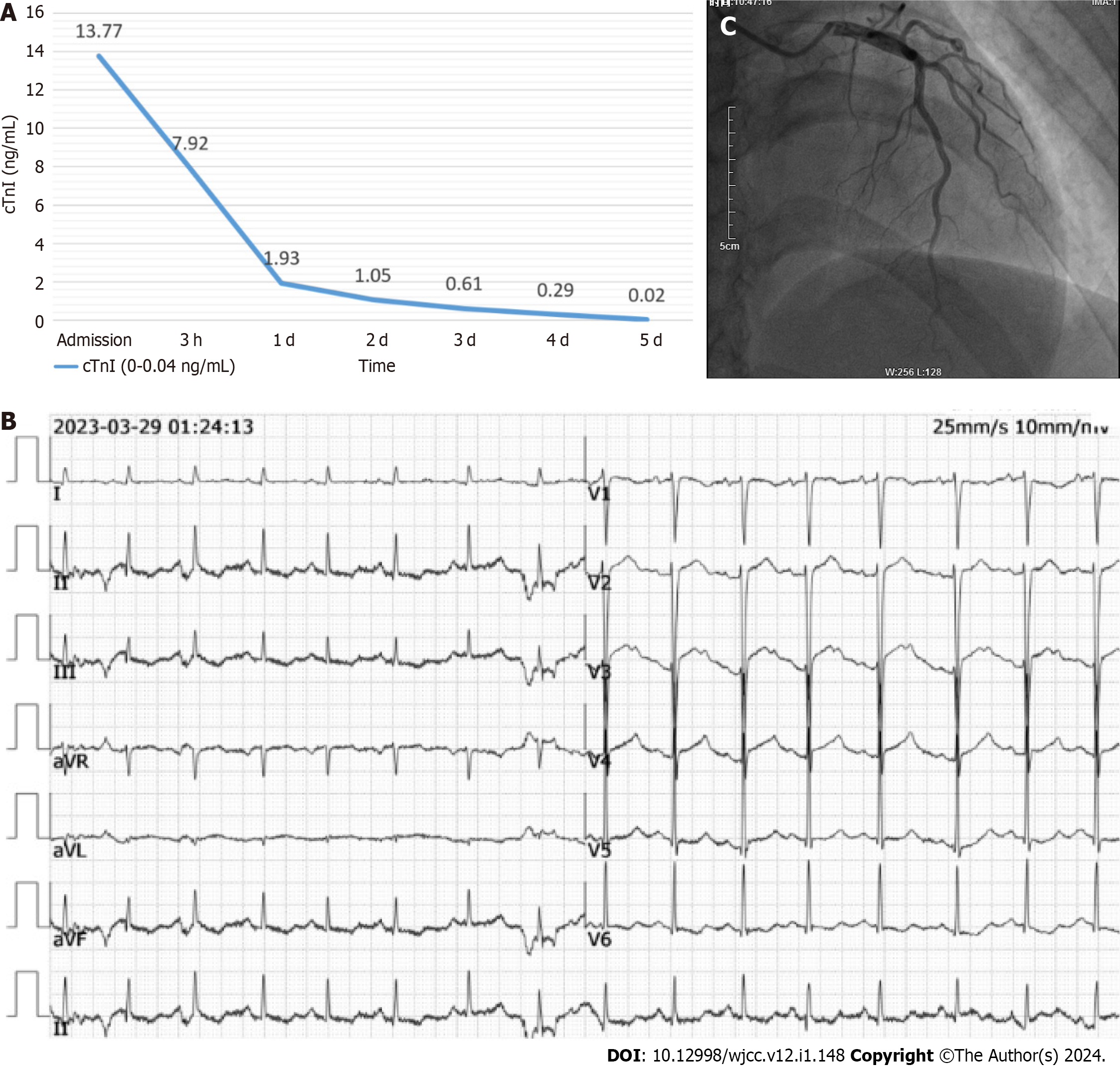
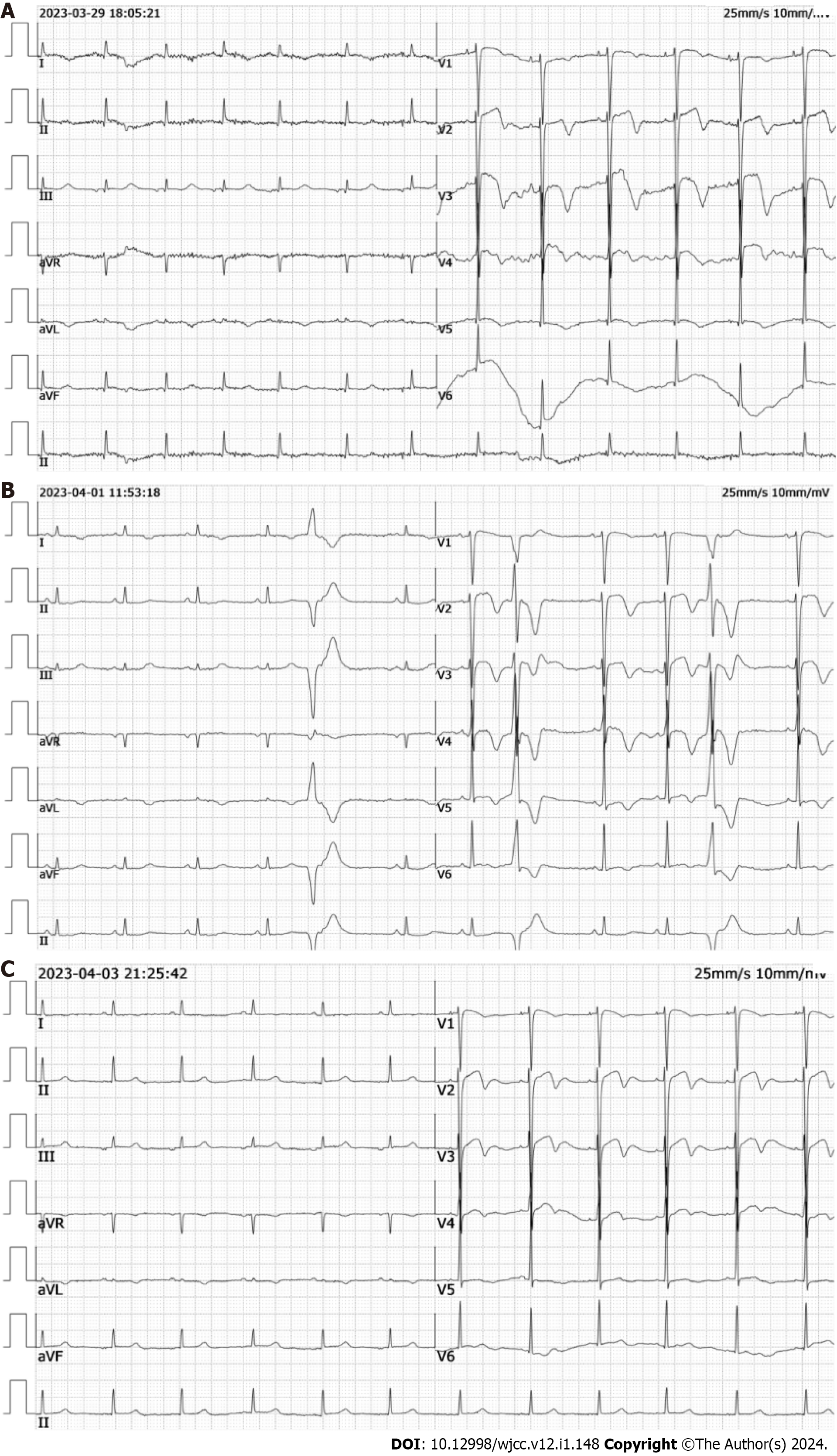
Coagulation function was normal, troponin I was 13.77 ng/mL (reference range: 0-0.04 ng/mL) (Figure 1A), and electrocardiogram suggested an ST elevation of 2 mm in the V2, V3, and V4 leads (Figure 1B). Cardiac ultrasound showed a hypo-diastolic left ventricle with a 68% ejection fraction. The initial diagnosis was ACS due to extremely high troponin levels and ST-segment elevation on the electrocardiogram. Therefore, the patient was admitted to the Department of Cardiology.
Considering the relative hemodynamic stability at the time of admission, oral antiplatelet therapy (aspirin and clopidogrel) was initiated, and coronary angiography (during which a high dose of low molecular heparin was used) was arranged; coronary angiography showed no significant vasospasm, especially in the left anterior descending coronary artery (Figure 1C).
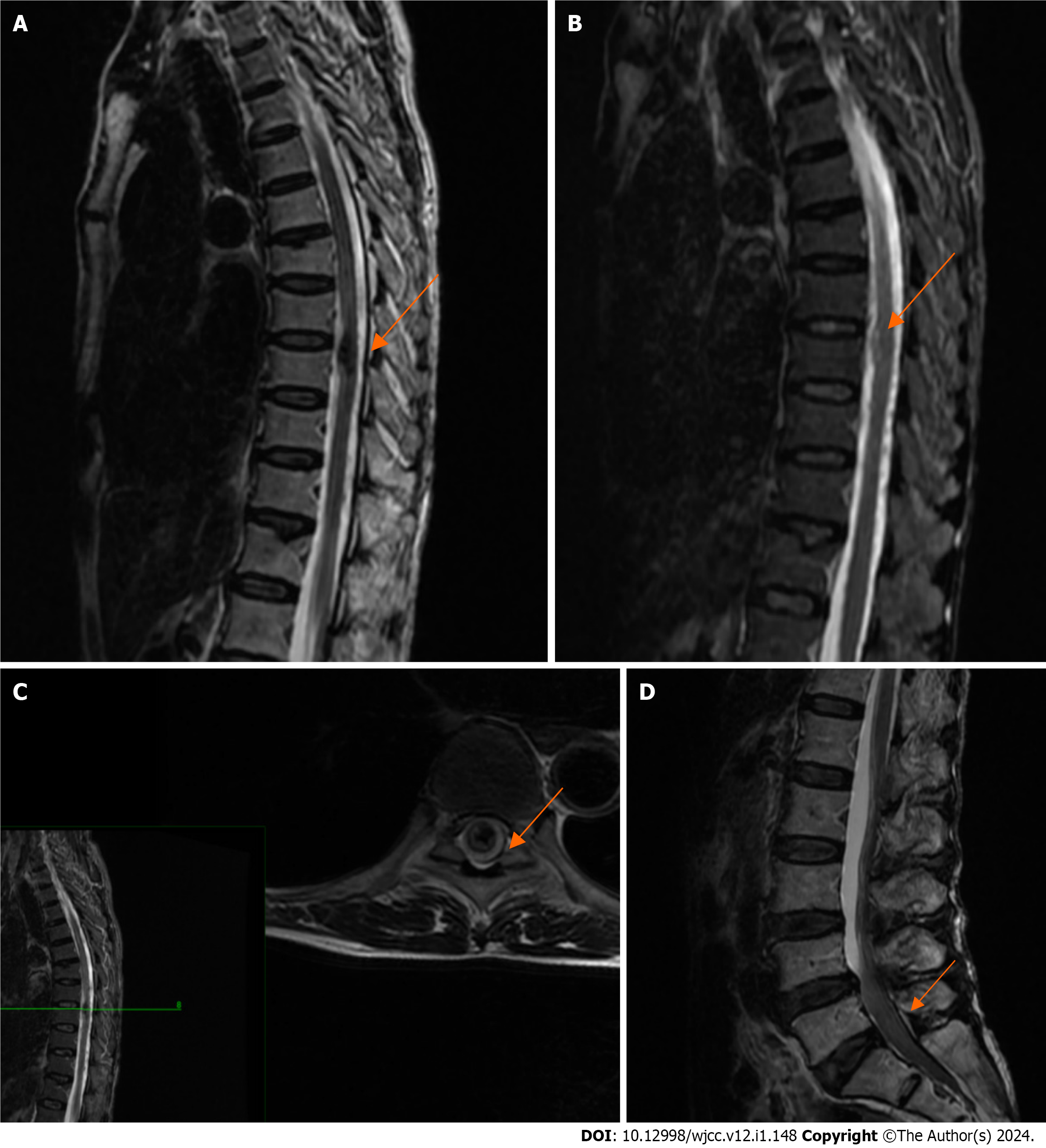
After coronary angiography showed no significant abnormality, a magnetic resonance imaging (MRI) scan of the thoracic and lumbar spine was arranged for the patient to exclude spinal disease, and the results suggested intramedullary hemorrhage in the T9 vertebral body and signal abnormality in the L4-S2 spinal canal (Figure 2).
The final diagnosis was intraspinal hematoma in the thoracic spine.
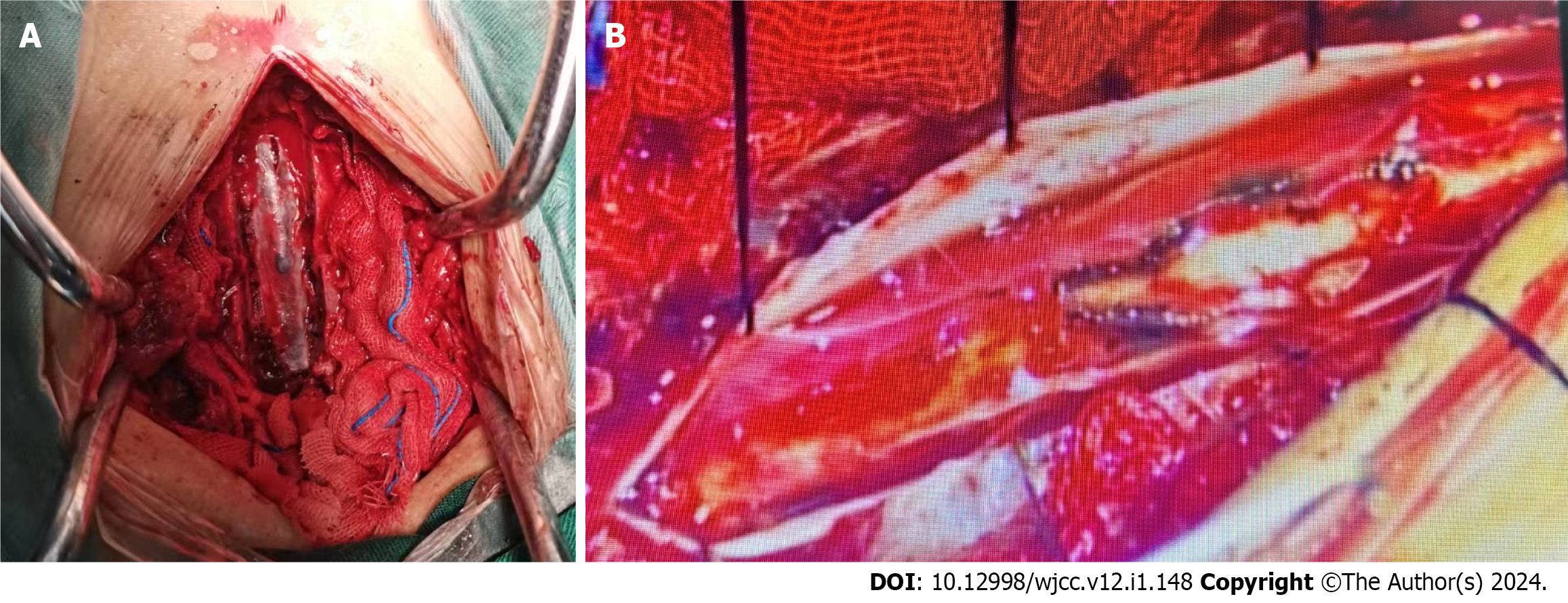
Due to the potential increased risk of bleeding, the patient was immediately taken off antiplatelet drug therapy. Surgical treatment was advised; however, the patient's family temporarily declined surgery and opted for conservative treatment. In the evening, the patient suddenly experienced worsening neurological symptoms, with a muscle strength of 0 in the lower extremities, bilateral sensory loss below the T12 rib margin, and a VAS score of 7. We performed an emergency thoracic spinal canal hematoma evacuation procedure with the assistance of a microscope. Intraoperatively, diffuse hematoma formation was found in the T7-T10 spinal canal, and no obvious spinal vascular malformation changes were observed (Figure 3A). We identified ruptured small blood vessels in the spinal arachnoid membrane under the microscope and used bipolar electrocoagulation for hemostasis (Figure 3B).
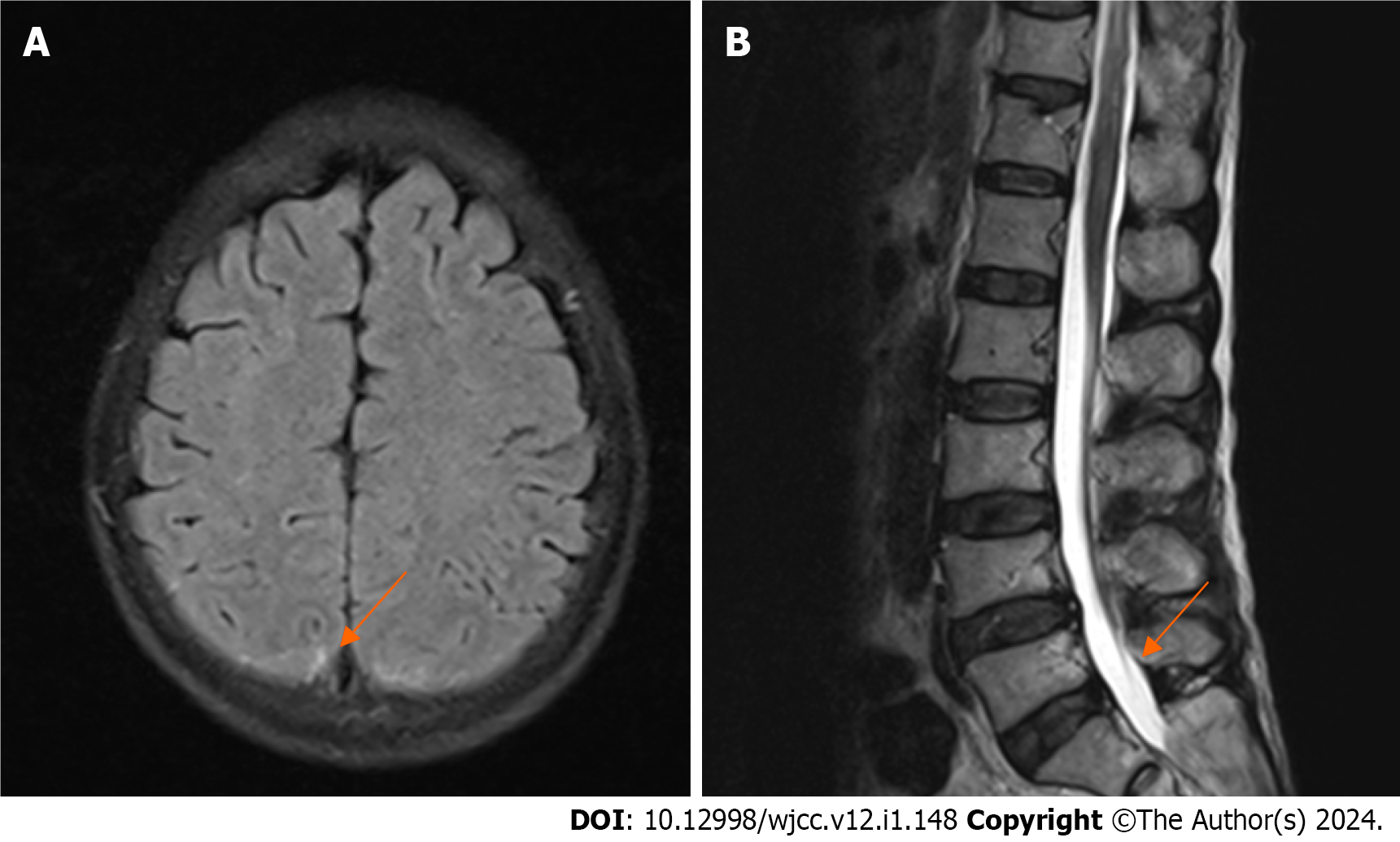
Postoperative head and spinal MRI showed a small amount of SAH in the skull, no obvious abnormalities in the cervical and thoracic spinal canals, and no abnormal signals in the lumbar spinal canal (Figure 4). Thoracoabdominal aorta computed tomography angiography showed no vascular malformation. Follow-up electrocardiogram showed ST-segment changes, T-wave inversion, and no pathological Q waves (Figure 5). The follow-up troponin I test was normal (Figure 1A). Postoperative motor system examination showed MRC grade 1/5 strength in both lower extremities, and the patient experienced decreased sensation below the T12 rib margin and reported a VAS score of 3.
Anatomically, the subdural space of the spinal cord is very narrow and lacks a bridging venous area compared to the subdural space of the skull, so a spinal subdural hematoma cannot occur on its own. Currently, it is believed that spontaneous spinal subdural hematoma (SSDH) is caused by hemorrhage in the subarachnoid space of the spinal cord[8,9]. Patients with hemorrhagic risk factors may experience a sudden increase in intrathoracic pressure due to sudden physical activity, leading to a sudden increase in vascular pressure within the thoracic spinal canal. When the pressure of the cerebrospinal fluid drops below the intravascular pressure, the venous structure of the spinal arachnoid may be torn, leading to a spinal subdural hematoma[8,9]. Our case is consistent with cases reported in the literature. During the surgery, we found subdural and subarachnoid hematomas spanning multiple segments of the spinal cord. The preoperative lumbar MRI of the patient suggested abnormal signals below L4, indicating a possible hematoma, which was completely absorbed at later follow-ups. The probable cause is the redistribution of the spinal cord hematoma due to the rapid dilution of the hematoma mixed with cerebrospinal fluid in the presence of an arachnoid tear[10]. This could also explain the postoperative signs of a small amount of SAH in our patient's skull. SSDH is most commonly found in the thoracic spine. It presents as sudden severe back pain (consistent with our case report) and causes varying degrees of motor, sensory, and autonomic dysfunction[11].
It is believed that 50% to 80% of patients with intracranial SAH will have electrocardiogram abnormalities[12]. The mechanism is thought to be increased sympathetic excitation after intracranial SAH, leading to increased release of catecholamines, resulting in impaired cardiac contraction and relaxation, abnormal repolarization, myocardial damage, and elevated cardiac biomarkers. Although electrocardiograms of intracranial SAH patients suggest myocardial ischemia, there is no evidence of coronary vasospasm, which is consistent with our case report[13,14]. ACS is a serious cardiovascular disease characterized by episodic chest pain, chest tightness, and other symptoms, with a high mortality rate[15]. Dual antiplatelet therapy, which consists of aspirin and a platelet P2Y12 receptor inhibitor (such as clopidogrel), is the main method of preventing ischemic events in the heart and throughout the body in patients with coronary heart disease with an increased risk of ischemia[16]. Due to abnormal changes in electrocardiogram and elevated cardiac biomarkers, SAH patients are often misdiagnosed with cardiovascular disease, leading to unnecessary cardiac function tests and wasted medical resources.
We report the case of a patient with spontaneous SAH of the spinal cord of unknown etiology who suffered catastrophic neurological damage after being misdiagnosed with ACS due to extremely high troponin levels and abnormal electrocardiogram findings and was treated with oral antiplatelet drugs. The patient had potential factors leading to an increase in thoracic pressure, and no evidence of spinal vascular malformation was found in the intraoperative or postoperative examinations. The patient had no history of coagulation disorders or anticoagulant use. Upon admission, the troponin level was 13.77 ng/mL (reference range: 0-0.04 ng/mL), and the electrocardiogram suggested a 2 mm ST elevation in leads V2, V3, and V4. The patient was misdiagnosed as having ACS and was treated with antiplatelet drugs. Furthermore, a high dose of low molecular weight heparin was used during coronary angiography, which is an absolute contraindication in patients with spinal SAH and may be related to the catastrophic neurological damage that occurred later. Patients with spinal hematomas may be considered for conservative treatment if symptoms are mild or begin to improve early[11]. This patient had mild early symptoms, and the family chose conservative treatment, which may have led to the poor prognosis.
Although the symptoms of ACS and spinal SAH can be similar, the treatment methods are completely opposite. If the symptoms of spontaneous spinal SAH are mild, conservative treatment can be considered. Surgery to remove the hematoma may be considered if neurological symptoms worsen, but the use of anticoagulant drugs is strictly prohibited[4]. If patients are misdiagnosed and given oral anticoagulants, catastrophic neurological damage may occur. We analyzed the causes of misdiagnosis. First, there have been many reports of intracranial SAH accompanied by cardiac abnormalities, but there are few reports on whether patients with spinal SAH also have concurrent cardiac abnormalities. The lack of understanding of spinal SAH may be a reason for our misdiagnosis. Second, unlike patients with ACS, whose troponin levels are usually up to 50 times the upper limit of the reference range within 2-4 h of onset, patients with intracranial SAH are believed to have only slightly increased troponin levels[7]. In our case, the patient's troponin level was more than 300 times the upper limit of the reference range, which is the first time that this has occurred in a patient with spinal SAH. In addition, it was previously thought that spinal vascular malformation, coagulation dysfunction, and treatment with anticoagulant or antiplatelet drugs were risk factors for spinal SAH and could lead to the misdiagnosis of other spontaneous spinal SAH cases of unknown etiology[17]. Last, in patients with severe back pain but no anterior chest pain who have extremely high troponin levels and abnormal changes in electrocardiogram, a diagnosis of spontaneous spinal SAH should also be considered. MRI can assist in the differential diagnosis when the diagnosis is unclear.
In our case, although the patient underwent timely hematoma removal surgery, she still recovered poorly from postoperative neurological symptoms, which seriously affected her quality of life. The preoperative use of two antiplatelet drugs and low molecular weight heparin may have been factors that exacerbated the patient's neurological damage. Based on our case, we suggest that extremely elevated troponin levels (more than 50 times outside the normal range) are not unique to coronary artery disease. Contrary to previous beliefs, spontaneous spinal SAH can also result in extremely high troponin levels (more than 300-fold above the normal range), and antiplatelet agents should not be used in such cases. Misdiagnosis of ACS can lead to catastrophic neurological damage in patients with spontaneous spinal SAH. The patient and her family refused further diagnosis and treatment and she was discharged after discussion.
Troponin levels 50-fold outside the normal range cannot be considered when differentiating between ACS and SAH, and misdiagnosis can have catastrophic consequences for such patients. We report, for the first time, the case of a patient with spontaneous spinal SAH of unknown etiology who suffered catastrophic neurological damage after being misdiagnosed with ACS. Emergency MRI aided in the early differential diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gokce E, Turkey S-Editor: Lin C L-Editor: Wang TQ P-Editor: Zhao S
| 1. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-e140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1953] [Cited by in F6Publishing: 2173] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 2. | Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4577] [Cited by in F6Publishing: 4223] [Article Influence: 703.8] [Reference Citation Analysis (1)] |
| 4. | Jung HS, Jeon I, Kim SW. Spontaneous Spinal Subdural Hematoma with Simultaneous Cranial Subarachnoid Hemorrhage. J Korean Neurosurg Soc. 2015;57:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Satyarthee GD, Ahmad F. Spontaneous Concurrent Intraspinal and Intracranial Subdural Hematoma: Management and Review of Literature. J Pediatr Neurosci. 2018;13:24-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 6. | Kunz U. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350-2354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Lee Y, Lim J, Han S, Choi SW, Youm JY, Koh HS. Spontaneous Spinal Subdural and Subarachnoid Hemorrhage with Concomitant Intracerebral Hemorrhage: A Case Report. Korean J Neurotrauma. 2019;15:34-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Gillilan LA. Veins of the spinal cord. Anatomic details; suggested clinical applications. Neurology. 1970;20:860-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 173] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Jung HS, Jeon I, Kim SW. Spontaneous Spinal Subdural Hematoma with Simultaneous Cranial Subarachnoid Hemorrhage. J Korean Neurosurg Soc. 2015;57:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kyriakides AE, Lalam RK, El Masry WS. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine (Phila Pa 1976). 2007;32:E619-E622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Oras J, Grivans C, Bartley A, Rydenhag B, Ricksten SE, Seeman-Lodding H. Elevated high-sensitive troponin T on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: a prospective observational study. Crit Care. 2016;20:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Wybraniec MT, Mizia-Stec K, Krzych Ł. Neurocardiogenic injury in subarachnoid hemorrhage: A wide spectrum of catecholamin-mediated brain-heart interactions. Cardiol J. 2014;21:220-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Junttila E, Vaara M, Koskenkari J, Ohtonen P, Karttunen A, Raatikainen P, Ala-Kokko T. Repolarization abnormalities in patients with subarachnoid and intracerebral hemorrhage: predisposing factors and association with outcome. Anesth Analg. 2013;116:190-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Bergmark BA, Mathenge N, Merlini PA, Lawrence-Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. 2022;399:1347-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 16. | Han Y. Chinese expert consensus statement on dual antiplatelet therapy in patients with coronary artery disease. Eur Heart J. 2022;43:1283-1285. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | de Beer MH, Eysink Smeets MM, Koppen H. Spontaneous Spinal Subdural Hematoma. Neurologist. 2017;22:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |