Copyright
©The Author(s) 2023.
World J Clin Cases. Jul 26, 2023; 11(21): 5083-5096
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
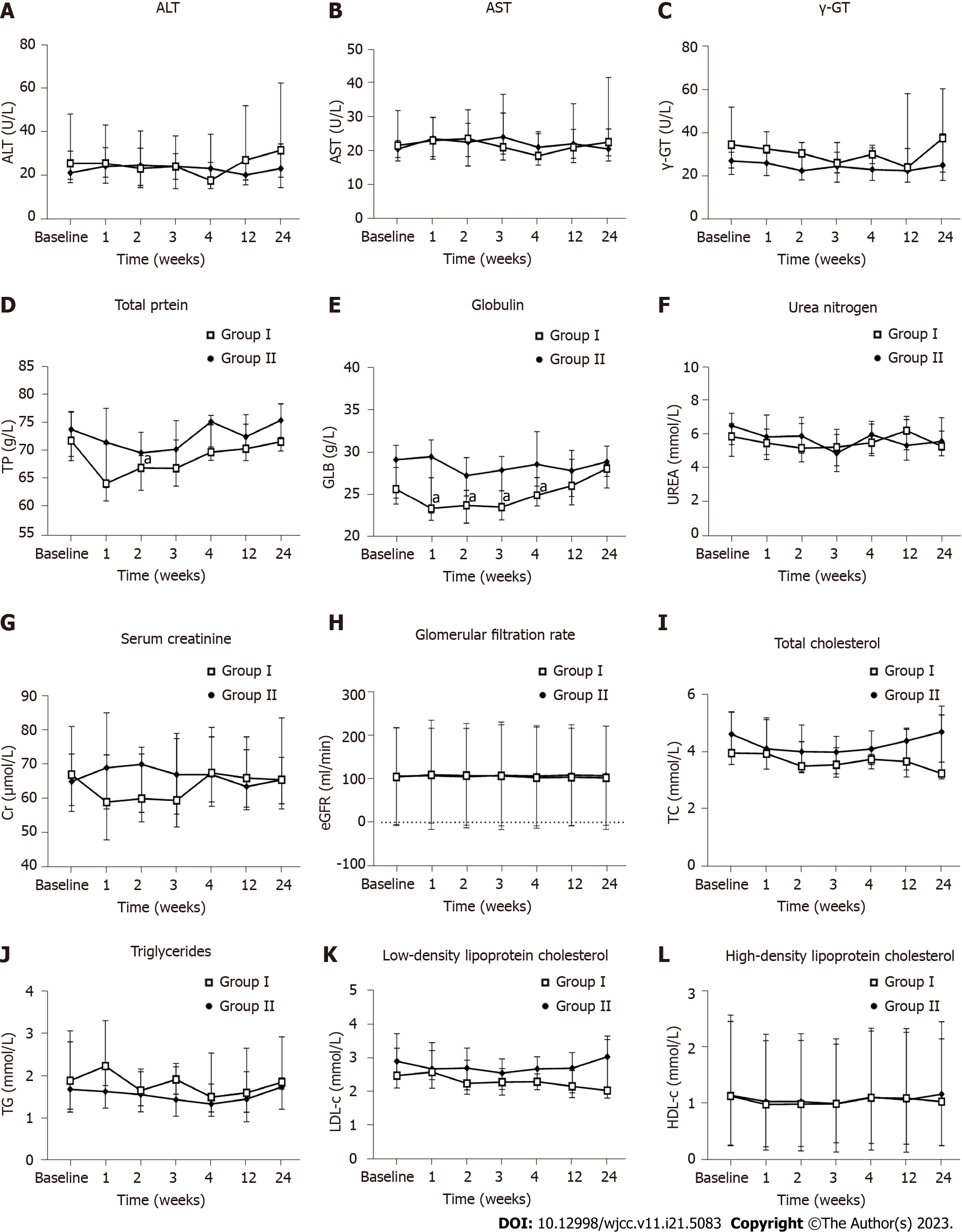
Figure 7 Influence of the human umbilical cord-mesenchymal stem cell treatment on liver function, renal function, and blood lipids.
Measurements for all indicators were taken at baseline and weeks 1, 2, 3, 4, 12, and 24 after the first infusion. A: Alanine transaminase; B: Aspartate transaminase; C: Gamma-glutamyl transferase; D: Total protein; E: Immunoglobulin; F: Urea nitrogen; G: Serum creatinine; H: Estimated glomerular filtration rate; I: Total cholesterol; J: Triglycerides; K: Low-density lipoprotein cholesterol; L: High-density lipoprotein cholesterol. aP < 0.05. Group I: Placebo group; Group II: Human umbilical cord-mesenchymal stem cell infusion group; ALT: Alanine transaminase; AST: Aspartate transaminase; γ-GT: Gamma-glutamyl transferase; TP: Total protein; GLB: Immunoglobulin; UREA: Urea nitrogen; Cr: Serum creatinine; eGFR: Estimated glomerular filtration rate; TC: Total cholesterol; TG: Triglycerides; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol.
- Citation: Lian XF, Lu DH, Liu HL, Liu YJ, Yang Y, Lin Y, Xie F, Huang CH, Wu HM, Long AM, Hui CJ, Shi Y, Chen Y, Gao YF, Zhang F. Safety evaluation of human umbilical cord-mesenchymal stem cells in type 2 diabetes mellitus treatment: A phase 2 clinical trial. World J Clin Cases 2023; 11(21): 5083-5096
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5083.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5083