Copyright
©The Author(s) 2023.
World J Clin Cases. Jul 26, 2023; 11(21): 5083-5096
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
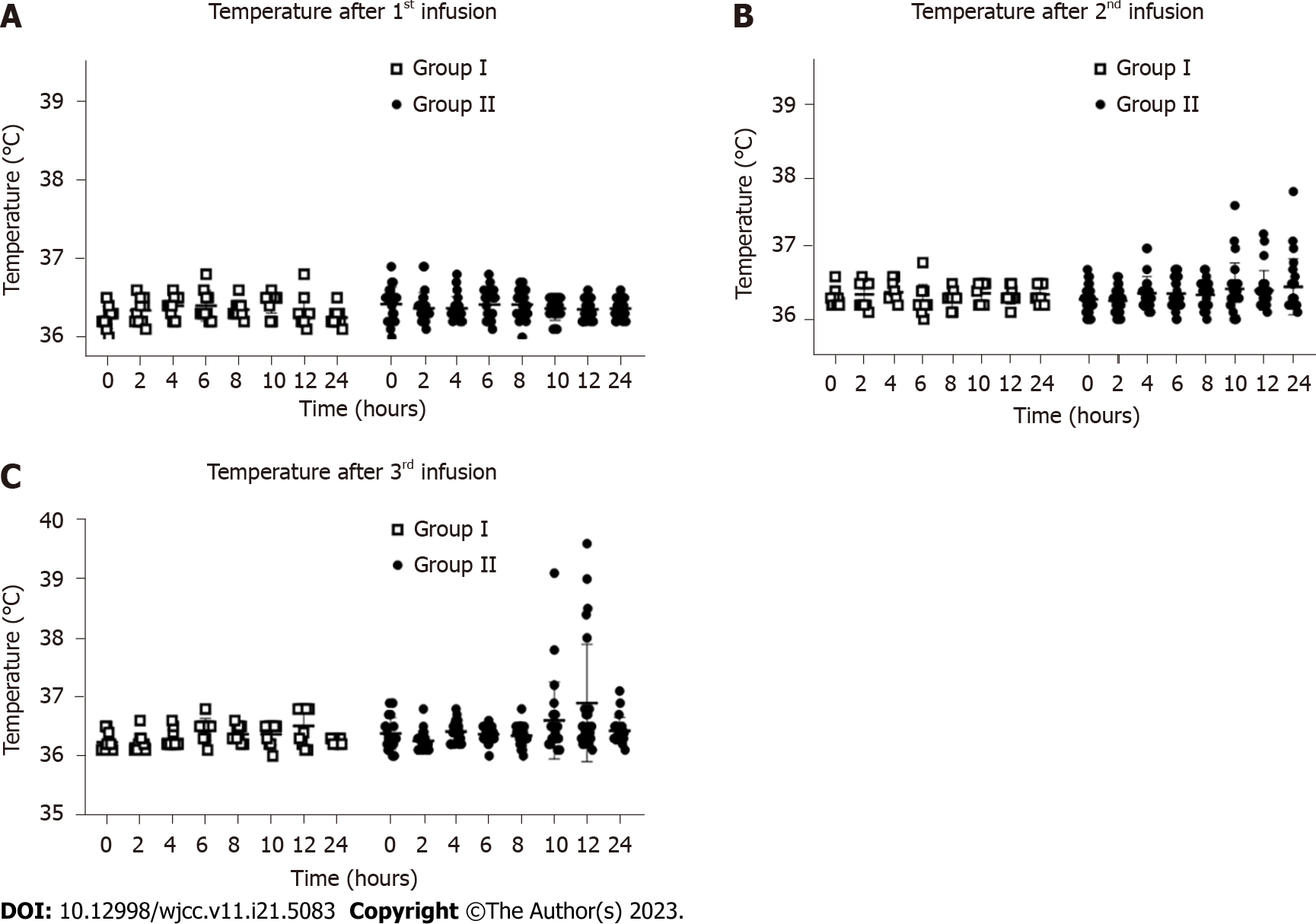
Figure 4 Fever occurrence within 24 h after infusion.
A: Temperatures of the patients within 24 h after the first infusion; B: Temperatures of the patients within 24 h after the second infusion; C: Temperatures of the patients within 24 h after the third infusion. Group I: Placebo group; Group II: Human umbilical cord-mesenchymal stem cell infusion group.
- Citation: Lian XF, Lu DH, Liu HL, Liu YJ, Yang Y, Lin Y, Xie F, Huang CH, Wu HM, Long AM, Hui CJ, Shi Y, Chen Y, Gao YF, Zhang F. Safety evaluation of human umbilical cord-mesenchymal stem cells in type 2 diabetes mellitus treatment: A phase 2 clinical trial. World J Clin Cases 2023; 11(21): 5083-5096
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5083.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5083