Copyright
©The Author(s) 2023.
World J Clin Cases. Jul 26, 2023; 11(21): 5083-5096
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5083
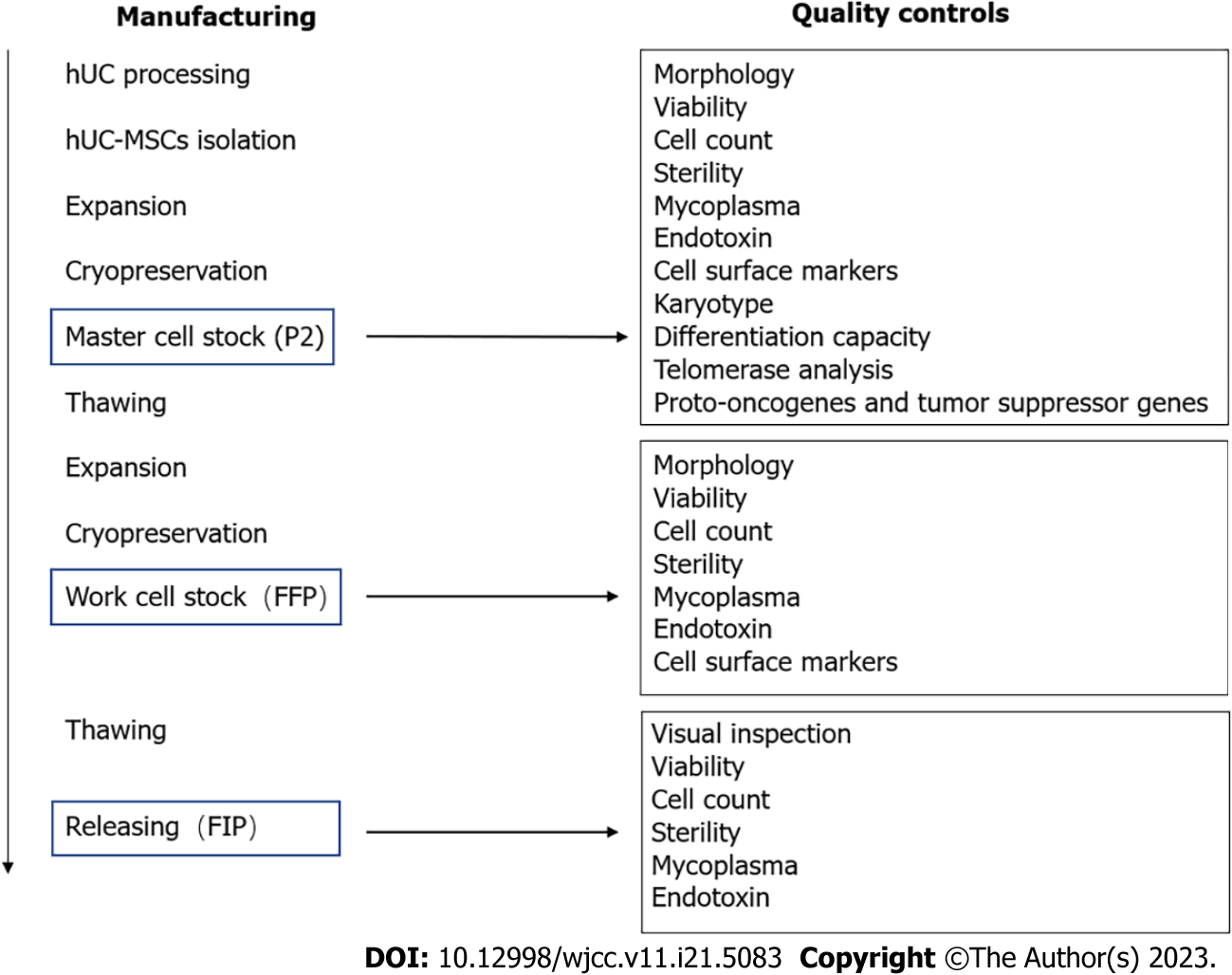
Figure 1 Stem cell manufacturing and quality control processes.
FFP: Final frozen product; FIP: Final infusion product; hUC: Human umbilical cord; MSCs: Mesenchymal stem cells; P2: Second passage.
- Citation: Lian XF, Lu DH, Liu HL, Liu YJ, Yang Y, Lin Y, Xie F, Huang CH, Wu HM, Long AM, Hui CJ, Shi Y, Chen Y, Gao YF, Zhang F. Safety evaluation of human umbilical cord-mesenchymal stem cells in type 2 diabetes mellitus treatment: A phase 2 clinical trial. World J Clin Cases 2023; 11(21): 5083-5096
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5083.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5083