Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4824
Peer-review started: June 6, 2023
First decision: June 15, 2023
Revised: June 16, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: July 16, 2023
Processing time: 35 Days and 21 Hours
Spinal osteoporosis is a prevalent health condition characterized by the thinning of bone tissues in the spine, increasing the risk of fractures. Given its high incidence, especially among older populations, it is critical to have accurate and effective predictive models for fracture risk. Traditionally, clinicians have relied on a combination of factors such as demographics, clinical attributes, and radiological characteristics to predict fracture risk in these patients. However, these models often lack precision and fail to include all potential risk factors. There is a need for a more comprehensive, statistically robust prediction model that can better identify high-risk individuals for early intervention.
To construct and validate a model for forecasting fracture risk in patients with spinal osteoporosis.
The medical records of 80 patients with spinal osteoporosis who were diagnosed and treated between 2019 and 2022 were retrospectively examined. The patients were selected according to strict criteria and categorized into two groups: Those with fractures (n = 40) and those without fractures (n = 40). Demographics, clinical attributes, biochemical indicators, bone mineral density (BMD), and radiological characteristics were collected and compared. A logistic regression analysis was employed to create an osteoporotic fracture risk-prediction model. The area under the receiver operating characteristic curve (AUROC) was used to evaluate the model’s performance.
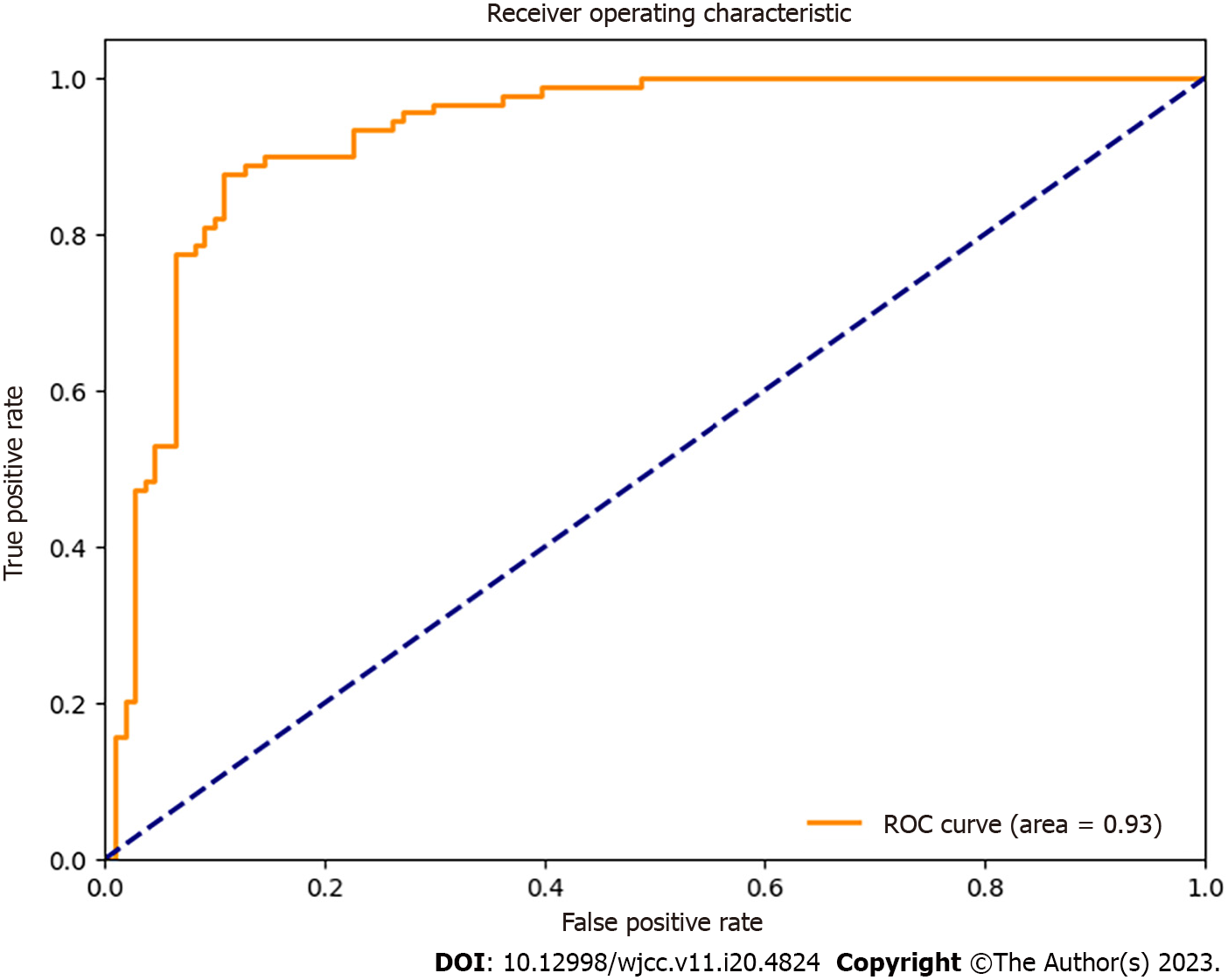
Factors significantly associated with fracture risk included age, sex, body mass index (BMI), smoking history, BMD, vertebral trabecular alterations, and prior vertebral fractures. The final risk-prediction model was developed using the formula: (logit [P] = -3.75 + 0.04 × age - 1.15 × sex + 0.02 × BMI + 0.83 × smoking history + 2.25 × BMD - 1.12 × vertebral trabecular alterations + 1.83 × previous vertebral fractures). The AUROC of the model was 0.93 (95%CI: 0.88-0.96, P < 0.001), indicating strong discriminatory capabilities.
The fracture risk-prediction model, utilizing accessible clinical, biochemical, and radiological information, offered a precise tool for the evaluation of fracture risk in patients with spinal osteoporosis. The model has potential in the identification of high-risk individuals for early intervention and the guidance of appropriate preventive actions to reduce the impact of osteoporosis-related fractures.
Core Tip: A fracture risk-prediction model was created and validated using the medical records of 80 patients with spinal osteoporosis. The model utilized accessible clinical, biochemical, and radiological information to accurately evaluate the patient's fracture risk. Factors significantly associated with fracture risk included age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures. The final model had strong discriminatory capabilities, as evidenced by an area under the receiver operating characteristic curve of 0.93. This model has potential in identifying high-risk individuals for early intervention and guiding appropriate preventive actions to reduce the impact of osteoporosis-related fractures.
- Citation: Lin XM, Shi ZC. Development and validation of a predictive model for spinal fracture risk in osteoporosis patients. World J Clin Cases 2023; 11(20): 4824-4832
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4824.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4824
Osteoporosis, a chronic systemic skeletal disease, poses a significant public health burden worldwide, affecting millions of people, particularly the elderly[1]. Characterized by diminished bone mass, degeneration of the bone tissue microarchitecture, and reduced bone strength, osteoporosis predisposes individuals to an increased risk of fractures[2]. Importantly, osteoporosis-related fractures can result in various complications, including chronic pain, disability, decreased quality of life, and elevated mortality rates[3].
Spinal osteoporosis, a subset of osteoporosis, is a prevalent condition that particularly affects the trabecular bones of the vertebrae[4]. The trabecular bone is responsible for biomechanical load-bearing in the spine, and deterioration of its structure increases the likelihood of vertebral fracture[5]. Vertebral fractures are the most common type of osteoporotic fractures and can occur even with minimal trauma or daily activities due to the weakened bone structure[2,6]. In addition to acute pain, vertebral fractures can lead to long-term consequences, such as kyphosis (abnormal curvature of the spine), decreased lung function, gastrointestinal complications, increased risk of subsequent fractures, and impaired mobility, all of which collectively contribute to a decline in overall health and well-being[7,8].
Early detection of those at an increased risk of fractures is essential for implementing proper preventive and therapeutic strategies and reducing the healthcare burden linked to fractures caused by osteoporosis[9]. Several clinical tools have been devised to predict the risk of osteoporotic fractures, with the fracture risk assessment tool (FRAX) being the most common. Developed by the World Health Organization, FRAX predicts a 10-year probability of hip and significant osteoporotic fractures based on age, sex, weight, height, and previous fracture history[10]. However, since it does not consider the unique features of spinal osteoporosis, such as changes in the vertebral body or trabecular bone microarchitecture, FRAX has limited effectiveness in the specific prediction of fracture risk in patients with spinal osteoporosis[11].
In recent years, there has been a growing interest in exploring alternative approaches for the accurate and precise prediction of fracture risk in patients with spinal osteoporosis. For example, previous studies have investigated readily available clinical, biochemical, and imaging data to examine their predictive value beyond that provided by traditional risk factors and bone mineral density (BMD) assessment[12,13]. Additionally, novel imaging techniques using magnetic resonance imaging (MRI) and computed tomography (CT) have shown potential for the quantification of vertebral microarchitecture and the assessment of its relation to fracture risk[14,15]. Furthermore, several studies have demonstrated that patients with spinal osteoporosis exhibit specific patterns of clinical risk factors and radiological findings that can help identify those at higher risk of fractures[16,17]. However, despite these advances, no comprehensive and validated fracture risk-prediction model has been developed for spinal osteoporosis patients. A model of this type that considers relevant demographic, clinical, biochemical, and radiological factors would provide this population with more accurate and personalized risk estimates, thereby enabling better-targeted interventions to prevent fractures and their associated complications.
In this study, we aimed to develop and validate a novel fracture risk-prediction model that integrates demographic, clinical, biochemical, and radiological factors to estimate the risk of osteoporotic fractures in patients with spinal osteoporosis. We hypothesized that this model would significantly improve the accuracy of risk prediction compared to traditional models, such as FRAX. The ultimate goal was to provide a practical and effective tool that clinicians can use to identify high-risk patients with spinal osteoporosis, guiding the implementation of appropriate preventive measures and management strategies to reduce the burden of osteoporosis-related fractures.
This retrospective cohort study was conducted at a tertiary clinic outfitted with a specialized osteoporosis treatment center. The protocol was reviewed and approved by the hospital’s ethics board. Informed consent was unnecessary due to the anonymizing of patient records and the study’s retrospective nature.
The medical records of patients with spinal osteoporosis who were treated at our institution between January 2019 and December 2022 were evaluated. Patients older than 50 years, a diagnosis of spinal osteoporosis according to the World Health Organization’s diagnostic standards, a T-score of -2.5 or less at the lumbar spine as determined by dual-energy X-ray absorptiometry (DXA), and with a complete record of clinical, biochemical, and imaging data were included. Patients who presented with secondary causes of osteoporosis, including hyperparathyroidism, hypogonadism, rheumatoid arthritis, chronic kidney disease, cancer, or had a history of spinal surgery or osteoporosis medications before diagnosis, were excluded from this study.
Based on the presence or absence of fractures, the 80 qualifying spinal osteoporosis patients who met the inclusion and exclusion requirements were divided into a fracture group (n = 40) and a non-fracture group (n = 40). Osteoporotic fractures were determined by identifying fragility fractures incurred from low-impact accidents, such as those resulting from a fall from a standing position.
Patient data, including demographics, clinical attributes, laboratory findings, and imaging results, were collected from electronic health records. The following is a detailed explanation of the included variables.
Demographic data: Age, sex, and body mass index (BMI, computed as weight in kilograms divided by height in meters squared) data were recorded.
Clinical attributes: Data relating to smoking history (defined as individuals who had smoked more than 100 cigarettes in their lifetime), alcohol consumption (defined as individuals who consumed at least one standard drink per week), and glucocorticoid usage (defined as a cumulative dose equivalent to at least 5 mg of prednisolone daily for three months or longer) were collected.
Biochemical markers: Serum calcium, phosphate, alkaline phosphatase, 25-hydroxyvitamin D[25(OH)D], and para
Bone mineral density (BMD): Lumbar spine BMD (L1–L4) measurements were conducted by DXA using a Hologic scanner (Hologic, Inc., Marlborough, MA, USA). Densitometry technologists who were certified according to the International Society for Clinical Densitometry’s standardized protocols performed and evaluated all BMD measurements. The BMD values were presented in absolute terms (g/cm²) and T-scores, representing the number of standard deviations from the average BMD for a young, sex-matched reference population.
Radiographical characteristics: (1) Vertebral trabecular alterations: A trained radiologist, blinded to the patient’s clinical information and fracture history, conducted a qualitative examination of vertebral trabecular changes. MRI or CT scans acquired as part of routine osteoporosis evaluations or for other clinical reasons were used to assess the trabecular changes. The presence or absence of trabecular alterations, such as trabecular loss or disturbance, was documented for each subject; and (2) Previous vertebral fractures: The occurrence or absence of previous vertebral fractures was determined by examining spinal radiographs (anteroposterior and lateral views). The Genant semi-quantitative method[18] was used to classify fractures according to the reduction in vertebral body height as grade 1 (mild), representing a 20%-25% reduction, grade 2 (moderate) indicating a 25%-40% reduction, and grade 3 (severe) indicating more than 40% reduction.
All statistical analyses were conducted using SPSS version 22.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics were expressed as means ± SD for continuous variables and percentages for categorical variables. To analyze differences between the fracture and non-fracture groups, Student’s t-tests or Mann-Whitney U tests were used for continuous variables, and chi-square or Fisher’s exact tests were used for categorical variables. A P value < 0.05 was considered statistically significant.
Univariate logistic regression analyses were utilized to investigate the connections between individual factors and the risk of fractures. Variables with a P value < 0.10 in the univariate analyses were included in the multivariate logistic regression model, and backward elimination was employed to identify independent predictors of fracture risk. The Akaike information criterion was utilized for model selection. The risk prediction model was developed based on the final regression coefficients of the significant predictors.
The performance of the risk prediction model was evaluated using the area under the receiver operating characteristic curve (AUROC). An AUROC value of > 0.70 was considered acceptable, while an AUROC value of > 0.80 was considered excellent[18,19]. Confidence intervals (CI) for the AUROC were estimated using the Delong method. To assess the model’s calibration, the observed and predicted probabilities of fractures were plotted on a calibration curve and compared.
The average age of the study cohort was 64.3 ± 12.2 years, with the majority being female (55.0%). No notable differences in alcohol consumption or glucocorticoid usage between the fracture and non-fracture groups were observed. However, the fracture group had a significantly higher percentage of patients with a history of smoking (47.5% vs 22.5%; P = 0.011) and pre-existing vertebral fractures (57.5% vs 20.0%; P < 0.001) than the non-fracture group. Additionally, the fracture group exhibited a lower average BMI (22.8 ± 3.5 kg/m² vs 24.6 ± 3.7 kg/m²; P = 0.004) and lumbar spine BMD (0.70 ± 0.13 g/cm² vs 0.85 ± 0.10 g/cm²; P < 0.001) compared to the non-fracture group (Table 1).
| Characteristics | Fracture group (n = 40) | Non-fracture group (n = 40) | P value |
| Age (mean ± SD, yr) | 67.5 ± 11.8 | 61.2 ± 12.2 | 0.024 |
| Gender (Female) | 22 (55.0) | 22 (55.0) | 1.000 |
| BMI (mean ± SD, kg/m²) | 22.8 ± 3.5 | 24.6 ± 3.7 | 0.004 |
| Smoking history | 19 (47.5) | 9 (22.5) | 0.011 |
| Alcohol consumption | 7 (17.5) | 9 (22.5) | 0.799 |
| Glucocorticoid use | 4 (10.0) | 3 (7.5) | 0.737 |
| BMD (mean ± SD, g/cm²) | 0.70 ± 0.13 | 0.85 ± 0.10 | < 0.001 |
| Vertebral trabecular changes | 28 (70.0) | 18 (45.0) | 0.021 |
| Previous vertebral fractures | 23 (57.5) | 8 (20.0) | < 0.001 |
The univariate logistic regression analysis revealed that age, sex, BMI, smoking history, BMD, vertebral trabecular alterations, and prior vertebral fractures contributed to an elevated risk of osteoporotic fractures. These factors remained significant in the multivariate regression analysis and were integrated into the final risk prediction model (Table 2).
| Variables | Univariable OR (95%CI) | P value | Multivariable OR (95%CI) | P value |
| Age (yr) | 1.04 (1.00-1.08) | 0.044 | 1.04 (1.00-1.08) | 0.034 |
| Gender (Female) | 0.62 (0.27-1.42) | 0.258 | 0.32 (0.12-0.86) | 0.022 |
| BMI (kg/m²) | 0.85 (0.76-0.95) | 0.003 | 0.88 (0.78-1.00) | 0.046 |
| Smoking history | 3.09 (1.29-7.41) | 0.011 | 2.30 (0.87-6.10) | 0.092 |
| BMD (g/cm²) | 0.01 (0.00-0.10) | < 0.001 | 0.02 (0.01-0.21) | 0.001 |
| Vertebral trabecular changes | 2.80 (1.22-6.44) | 0.016 | 4.29 (1.61-11.41) | 0.004 |
| Previous vertebral fractures | 5.37 (2.12-13.60) | < 0.001 | 3.83 (1.59-9.22) | 0.003 |
Drawing from the multivariate regression analysis, the risk prediction model for osteoporotic fractures in individuals with spinal osteoporosis was constructed as follows: logit (P) = -3.75 + 0.04 × age - 1.15 × sex + 0.02 × BMI + 0.83 × smoking history + 2.25 × BMD - 1.12 × vertebral trabecular alterations + 1.83 × previous vertebral fractures.
The model incorporated age (in years), sex (1 for male, 0 for female), BMI (in kg/m²), smoking history (1 for yes, 0 for no), lumbar spine BMD (in g/cm²), vertebral trabecular alterations (1 for yes, 0 for no), and the presence of previous vertebral fractures (1 for yes, 0 for no).
The AUROC of the risk prediction model was 0.93 (95%CI: 0.88-0.96, P < 0.001), indicating good discriminatory performance (Figure 1). The model demonstrated satisfactory calibration, with observed vs predicted probabilities not deviating significantly from the ideal (Figure 2).
In this study, we developed and validated a fracture risk-prediction model specifically designed for patients with spinal osteoporosis using demographic, clinical, biochemical, and radiological factors. The model demonstrated good discriminatory performance (AUROC = 0.93) and calibration, suggesting its potential usefulness for the accurate identification of high-risk patients and the guidance of appropriate preventive measures and treatment options tailored for this population.
Our findings corroborated those of previous research on osteoporotic fractures by showing a significant contributing role for age, sex, BMI, and smoking history as important clinical predictors of fracture risk[20,21]. These factors have been well established as risk factors for fractures in the general population; however, this study confirms their specific association with fracture risk in patients with spinal osteoporosis. The present study provides additional evidence that these factors should be considered when assessing fracture risk in this population.
Interestingly, our model also incorporated lumbar spine BMD, vertebral trabecular changes, and the presence of previous vertebral fractures, which were found to be significant predictors of fracture risk in the study population. While lumbar spine BMD has long been recognized as a valuable parameter for assessing fracture risk[22], the inclusion of vertebral trabecular changes and previous fractures in the present model emphasizes the need to consider specific radiological findings in patients with spinal osteoporosis. Vertebral trabecular changes, assessed using advanced imaging techniques such as MRI or CT, provide valuable insights into the structural deterioration and the quality of the trabecular bone, which are not captured by BMD measurements alone[23,24]. Furthermore, a history of previous vertebral fractures may be a crucial risk factor, as these often indicate underlying structural weaknesses and predispose patients to further fractures[25,26]. Our findings support the importance of considering these unique characteristics when estimating the fracture risk in spinal osteoporosis patients.
The strength of our study lies in the development of a prediction model specifically tailored to patients with spinal osteoporosis. The model incorporated a combination of easily obtainable demographic, clinical, and biochemical variables and key information from radiological assessments (i.e., vertebral trabecular changes and previous vertebral fractures). In comparison, the widely used FRAX tool does not directly consider these specific features of spinal osteoporosis[27]. Therefore, our model may offer a more accurate assessment of fracture risk for this patient population and could be an essential tool in the comprehensive assessment and management of spinal osteoporosis.
Our study has several limitations that should be acknowledged. First, the retrospective nature of the analysis and the relatively small sample size drawn from a single institution may limit the generalizability of our findings. Larger prospective studies are needed to confirm the external validity of our risk prediction model before it can be widely implemented. Second, the analysis did not include potentially relevant variables such as physical activity levels, dietary factors, or detailed medication history. Future research should explore the inclusion of such variables to refine the model further and improve its predictive performance. Third, our study relied on radiological assessments of vertebral trabecular changes and previous vertebral fractures, necessitating access to advanced imaging techniques such as MRI or CT scans and expert radiological interpretation. Implementing this in routine clinical practice may be challenging, especially in resource-limited settings. Future research could explore alternative imaging techniques or simplified assessment methods to address this issue, which could be more conveniently implemented in daily clinical practice.
Despite these limitations, our study contributes to the growing literature on spinal osteoporosis and fracture risk prediction. The accurate prediction of fracture risk is crucial for the early identification of high-risk individuals and the optimal allocation of resources, and the accurate assessment of fracture risk in patients with spinal osteoporosis has important implications for individualized patient management and treatment decisions.
In conclusion, our study presents a novel fracture risk prediction model for patients with spinal osteoporosis using readily available demographic, clinical, biochemical, and radiological data. This model offers an accurate tool to estimate the risk of osteoporotic fractures, helping identify high-risk patients for early intervention and guiding appropriate preventive measures to reduce the healthcare burden associated with osteoporosis-related fractures. This tool will help facilitate clinicians’ decisions regarding managing spinal osteoporosis and improve patient outcomes.
Spinal osteoporosis is a prevalent condition that increases the risk of fractures, particularly among older populations. Traditional predictive models for fracture risk rely on a limited set of factors and lack precision. This study aimed to develop and validate a more comprehensive prediction model that includes a wider range of factors, such as biochemical indicators and bone mineral density, based on the medical records of 80 patients with spinal osteoporosis. Factors significantly associated with fracture risk included age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures. The final risk-prediction model demonstrated strong discriminatory capabilities and has potential for identifying high-risk individuals for early intervention and guiding appropriate preventive actions.
The high incidence of spinal osteoporosis among older populations and the associated risk of fractures highlights the need for accurate and effective predictive models for fracture risk. However, traditional models have limitations in terms of precision and inclusiveness of potential risk factors. This study was motivated by the need to develop a more comprehensive and statistically robust prediction model that can better identify high-risk individuals for early intervention. By incorporating a wide range of factors, including biochemical indicators and bone mineral density, the developed model offers a precise tool for evaluating fracture risk in patients with spinal osteoporosis. The model has the potential to significantly improve patient outcomes by enabling early intervention and guiding appropriate preventive actions to reduce the impact of osteoporosis-related fractures. Ultimately, this research is driven by the goal of improving the quality of life for patients with spinal osteoporosis and reducing the burden of osteoporosis-related fractures on individuals and healthcare systems.
The main objective of this research was to develop and validate a prediction model for spinal fracture risk in patients with osteoporosis. The model aimed to offer improved accuracy and inclusiveness of potential risk factors compared to traditional models, which often lack precision and fail to consider all relevant variables. To achieve this objective, the study employed a retrospective analysis of medical records from 80 patients with spinal osteoporosis. Demographic, clinical, biochemical, and radiological data were collected and compared between patients who had experienced fractures and those who had not. Using logistic regression analysis, the study identified factors significantly associated with fracture risk, including age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures. Based on these findings, the researchers developed a final prediction model that incorporates multiple factors and demonstrated strong discriminatory capabilities in evaluating fracture risk. Overall, the study's objectives were to improve prediction accuracy and ultimately aid in early identification and intervention of individuals at high risk of osteoporosis-related fractures.
This study employed a retrospective analysis of medical records to develop and validate a prediction model for spinal fracture risk in patients with osteoporosis. The study included 80 patients with spinal osteoporosis who were diagnosed and treated between 2019 and 2022. Using strict criteria, the patients were categorized into two groups: Those with fractures (n = 40) and those without fractures (n = 40). Demographic, clinical, biochemical, and radiological data were collected from the medical records and compared between the two groups. A logistic regression analysis was employed to identify factors significantly associated with fracture risk. Based on these findings, a final prediction model was developed that incorporated multiple factors, including age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures. The model's performance was evaluated using the area under the receiver operating characteristic curve, which measures the model's discriminatory capabilities. The statistical analyses were conducted using appropriate software and tools. Overall, the study's methods involved a rigorous and systematic approach to developing an accurate and effective prediction model for spinal fracture risk in patients with osteoporosis.
Spinal osteoporosis is a common health condition that increases fracture risk. This study aimed to develop and validate a comprehensive model for predicting fracture risk in patients with spinal osteoporosis. The medical records of 80 patients were retrospectively analyzed, and factors associated with fracture risk, including age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures, were identified. Using logistic regression analysis, a risk-prediction model was developed and validated, showing strong discriminatory capabilities with an area under the receiver operating characteristic curve of 0.93. This model can potentially assist in identifying high-risk individuals for early intervention and guiding appropriate preventive actions to reduce the impact of osteoporosis-related fractures.
This study emphasizes the significance of a comprehensive approach to predicting fracture risk in patients with spinal osteoporosis. The developed model utilizing factors such as age, sex, body mass index, smoking history, bone mineral density, vertebral trabecular alterations, and prior vertebral fractures offers a precise tool for evaluating fracture risk in these patients. The model's strong discriminatory capabilities make it potentially valuable in identifying high-risk individuals for early intervention and guiding appropriate preventive measures to reduce the impact of osteoporosis-related fractures. By considering a wide range of accessible clinical, biochemical, and radiological information, clinicians can accurately assess fracture risk and improve patient outcomes.
This study's findings open up new research perspectives in the field of spinal osteoporosis. Further studies can focus on validating and refining the developed fracture risk-prediction model by including more comprehensive and diverse data sources, such as genetic factors and lifestyle behaviors. Additionally, larger-scale studies incorporating various ethnic groups and age ranges can provide insights into whether the developed model is applicable to wider populations. Furthermore, future research can evaluate the effectiveness of using the prediction model in clinical practice and determine its impact on reducing fracture-related morbidity and mortality rates. Lastly, considering the potential of artificial intelligence and machine learning algorithms in predicting fracture risk, there is scope for developing more sophisticated models that can identify high-risk individuals with higher accuracy and efficiency. In summary, this study provides a foundation for further research aimed at improving fracture risk prediction and prevention in patients with spinal osteoporosis.
In the process of researching and writing this manuscript, I would like to express my gratitude to all those who have helped me.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jadhav AP, United States; Shapiro M, United States S-Editor: Wang JL L-Editor: A P-Editor: Yu HG
| 1. | Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 1284] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 2. | Anam AK, Insogna K. Update on Osteoporosis Screening and Management. Med Clin North Am. 2021;105:1117-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C, Lorentzon M, McCloskey EV, Harvey NC, Javaid MK, Kanis JA; International Osteoporosis Foundation. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 4. | Qaseem A, Forciea MA, McLean RM, Denberg TD; Clinical Guidelines Committee of the American College of Physicians, Barry MJ, Cooke M, Fitterman N, Harris RP, Humphrey LL, Kansagara D, McLean RM, Mir TP, Schünemann HJ. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med. 2017;166:818-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 459] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 5. | Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, Saag K. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64:46-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Ensrud KE, Schousboe JT. Clinical practice. Vertebral fractures. N Engl J Med. 2011;364:1634-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | O'Connor KM. Evaluation and Treatment of Osteoporosis. Med Clin North Am. 2016;100:807-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 2395] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 10. | Arceo-Mendoza RM, Camacho P. Prediction of fracture risk in patients with osteoporosis: a brief review. Womens Health (Lond). 2015;11:477-82; quiz 483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA; Manitoba Bone Density Program. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, Cauley JA, Compston JE, Dawson-Hughes B, El-Hajj Fuleihan G, Johansson H, Leslie WD, Lewiecki EM, Luckey M, Oden A, Papapoulos SE, Poiana C, Rizzoli R, Wahl DA, McCloskey EV; Task Force of the FRAX Initiative. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 13. | Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012;263:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Krug R, Burghardt AJ, Majumdar S, Link TM. High-resolution imaging techniques for the assessment of osteoporosis. Radiol Clin North Am. 2010;48:601-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE; Fracture Intervention Trial Research Group. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 18. | Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2293] [Cited by in RCA: 2519] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 19. | Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken: Wiley, 2013. |
| 20. | Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1453] [Article Influence: 242.2] [Reference Citation Analysis (0)] |
| 21. | Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1699] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 22. | Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 2432] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 23. | Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1189] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 24. | Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 675] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 25. | Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA; IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 760] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 26. | Kanis JA, McCloskey EV, Harvey NC, Johansson H, Leslie WD. Intervention Thresholds and the Diagnosis of Osteoporosis. J Bone Miner Res. 2015;30:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1348] [Article Influence: 53.9] [Reference Citation Analysis (0)] |