Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4734
Peer-review started: February 17, 2023
First decision: May 18, 2023
Revised: May 31, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 16, 2023
Inflammatory myofibroblastic tumor (IMT) of the biliary tract is rare, and often difficult to diagnose or to distinguish from other tumors due to its atypical clinical presentation and nonspecific radiological features. Histologically, IMTs are (myo)fibroblastic neoplasms with a prominent inflammatory infiltrate. They are characterized by receptor tyrosine kinase gene rearrangements, most often involving an anaplastic lymphoma kinase (ALK) translocation. The final diagnosis of IMT depends on histopathology and immunohistochemical examination. In this manuscript, we provide a clinical and morphomolecular overview of IMT and the difficulties that may arise in using immunohistochemical and molecular techniques in diagnosing IMT.
Core Tip: Inflammatory myofibroblastic tumor (IMT) of the intrapancreatic biliary tract is rare and often difficult to diagnose. In this manuscript, we give a recent update of the clinicopathological features of IMT with focus on the pathological and molecular characteristics.
- Citation: Cordier F, Hoorens A, Ferdinande L, Van Dorpe J, Creytens D. Inflammatory myofibroblastic tumor of the distal common bile duct: Literature review with focus on pathological examination. World J Clin Cases 2023; 11(20): 4734-4739
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4734.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4734
Inflammatory myofibroblastic tumor (IMT) is a (myo)fibroblastic neoplasm with a prominent inflammatory infiltrate, consisting mainly of lymphocytes and plasma cells. Originally, IMT was reported in the lung by Brunn[1] but the term IMT was first proposed in 1990 by Pettinato et al[2]. It was regarded as an inflammatory pseudotumor until it was officially considered a separate entity by the World Health Organization (WHO) in 2002[3-5]. In the gastrointestinal tract, IMT occurs mainly in the small intestine and colon. It typically forms in the submucosa, muscularis propria or mesentery and gives rise to abdominal pain, intestinal obstruction or fever. IMT of the pancreas and biliary tract is extremely rare; few cases have been reported[4-7].
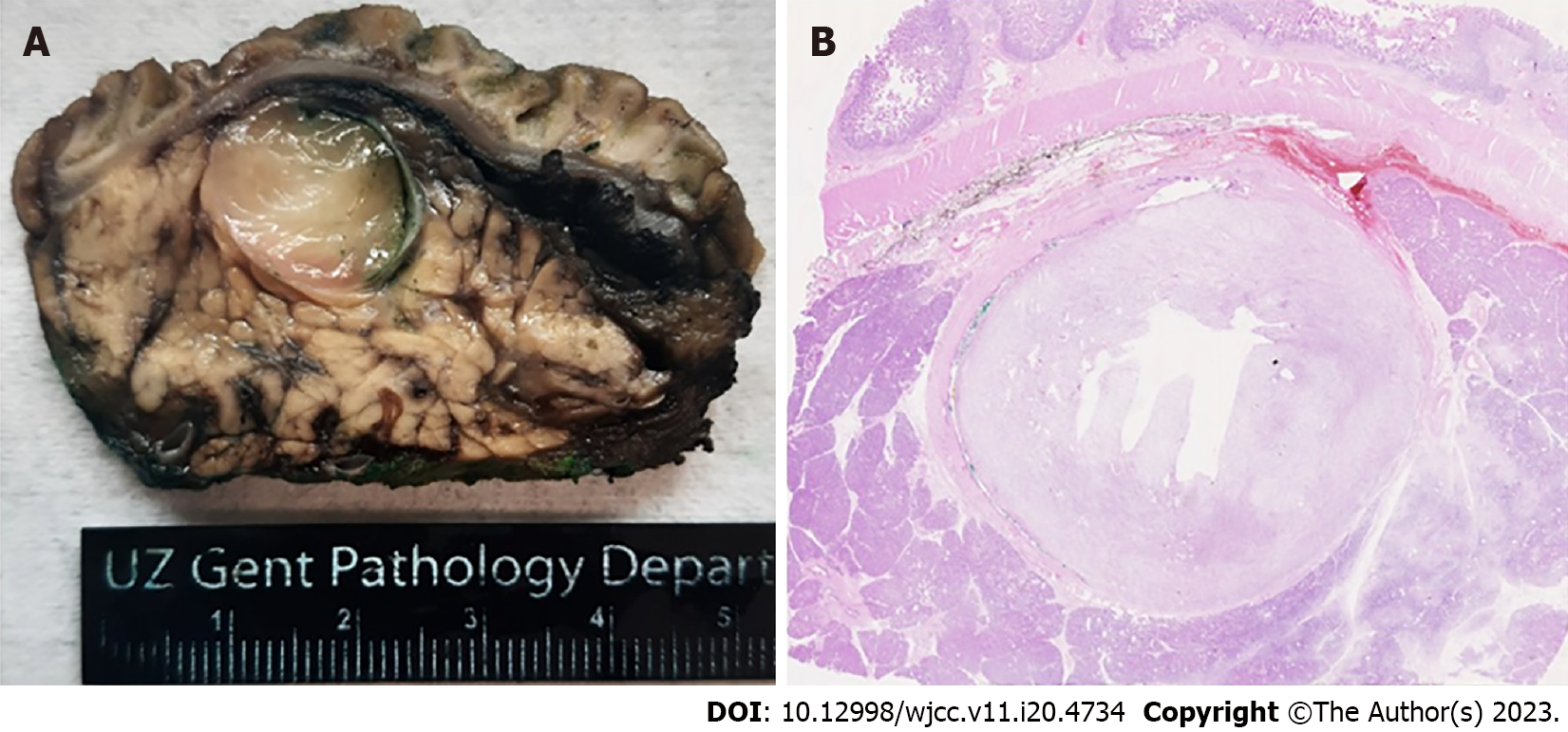
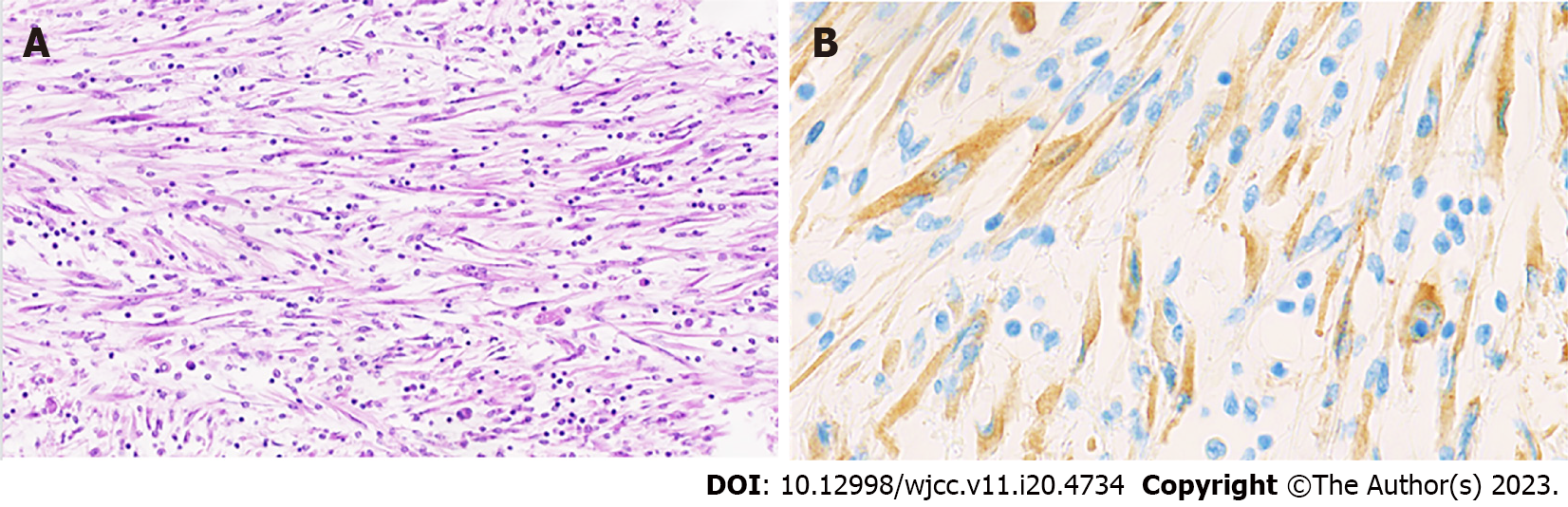
Recently, we encountered an IMT in the lumen of the distal common bile duct near the ampulla, in a 64-year-old woman. This lesion was discovered incidentally during follow-up imaging of the patient's metastatic breast carcinoma. Radiological examination revealed a mass, measuring approximately 17 by 14 mm, exerting pressure on the distal choledochus and resulting in bile duct dilatation of 11 mm. Interestingly, the patient did not exhibit any symptomatic signs related to this finding (no signs of obstructive jaundice). Clinically, there was suspicion of an ampullary carcinoma, leading to the decision to perform a Whipple resection. Macroscopically, a myxoid lesion was seen intrapancreatic, occupying the lumen of the common bile duct (2.1 cm × 1.6 cm) (Figure 1). Microscopical examination revealed an intraluminal mesenchymal lesion consisting of plump spindle cells with pale cytoplasm containing a vesicular nucleus. The stroma was myxoid with an inflammatory infiltrate composed of lymphocytes, plasma cells, macrophages and scarce eosinophils (Figure 2A). There was no necrosis or brisk mitotic activity. On immunohistochemistry (IHC), the tumor was negative for desmin, SOX10, S100, pancytokeratin AE1/AE3, DOG1 and CD34. IgG4/IgG ratio was normal. There was cytoplasmic immunohistochemical positivity for anaplastic lymphoma kinase (ALK) (Figure 2B), rendering the diagnosis of an IMT.
In this case, the histopathological differential diagnosis included gastrointestinal stromal tumor (GIST) and IgG4-related disease, which were ruled out by IHC. However, due to the exceptionally rare location of the lesion in the lumen of the common bile duct, additional fluorescence in situ hybridization (FISH) was performed to confirm the diagnosis of an IMT. Unfortunately, FISH could not confirm an ALK rearrangement, with only a split of signals in 12% of the counted tumor cells (equivocal result). Subsequently, RNA next-generation sequencing (NGS) was performed and detected an EML4::ALK fusion, confirming the diagnosis of IMT in our patient[8].
The age group for IMT is wide, but it usually occurs in children and young adults with no sex predilection[3-5,9,10]. In the pancreas, IMT usually occurs in the head of the pancreas and in the bile duct, it is more commonly seen in the hilus of the liver. It causes painless obstructive jaundice, abdominal pain, weight loss and fever[4,6,9-11].
Because of the rarity of IMT in the common bile duct or pancreatic head, its atypical clinical presentation and nonspecific radiological features, it is often difficult to distinguish IMT from other tumors. Therefore, most IMTs are surgically removed before definitive diagnosis[3-5,9,10].
IMTs may be solid, fleshy or gelatinous, with a white to yellowish-brown cut surface. In a minority of cases calcifications, bleeding and necrosis occur. The tumor size ranges from 1 cm to 20 cm, with an average of 6 cm[12-14].
Histologically, IMTs are composed of myofibroblastic spindle cells and inflammatory cells. Coffin et al[15] described three basic histological patterns: A myxoid/vascular pattern, a compact spindle cell pattern and a hypocellular fibrous (fibromatosis-like) pattern. These patterns are often seen in combination within the same tumor. The myxoid/vascular pattern has a fasciitis-like appearance, with loosely arranged plump spindle cells in an edematous or myxoid stroma and a prominent vasculature. The inflammatory infiltrate often demonstrates more neutrophils and eosinophils, and less plasma cells than in the other two patterns. The compact spindle cell pattern resembles fibrous histiocytoma with compact spindle cells intermixed by inflammatory cells (lymphocytes, plasma cells and eosinophils). The fibromatosis-like pattern is relatively hypocellular with a dense collagenous stroma showing scattered lymphocytes, plasma cells and eosinophils resembling a desmoid fibromatosis or scar[12,13,16].
The spindle cells of IMT are typically uniform and predominantly myofibroblastic. Mild nuclear pleomorphism may be seen, but hyperchromasia is absent[5,13]. About half of the cases contain scattered 'ganglion-like' cells. These are larger polygonal cells with abundant amphophilic to eosinophilic cytoplasm, large vesicular nuclei and prominent nucleoli, similar to those seen in proliferative fasciitis[12,14]. Mitotic activity is low (0–2 mitoses per 10 high power fields, and atypical mitoses are rare[5,12,13,15,17]. Necrosis and vascular invasion are rare, but can be observed[5,12,13,15,18]. Coffin et al[15] showed that the presence of necrosis, hypercellularity and ganglion-like cells was not related to clinical features, outcome or ALK reactivity. The presence of atypical mitoses should raise the possibility of an alternative diagnosis. In rare cases, IMT shows a higher-grade morphology with increased cellularity, epithelioid/histiocytoid or round cell morphology, marked nuclear atypia, frequent mitoses, atypical mitotic figures and/or necrosis[5,12,13,15,16,19-21]. This variant is referred to as epithelioid inflammatory myofibroblastic sarcoma (EIMS). EIMS occurs mainly intra-abdominal, is associated with a more aggressive course and shows a male predominance[5,7,16,19,22].
Immunohistochemically, IMTs demonstrate diffuse positivity for vimentin, muscle-specific actin and smooth muscle actin; and may show focal reactivity for cytokeratin, clearly showing the myofibroblastic nature of the tumor[5,13,15]. Staining for desmin and calponin is often focal[7,12,13,18]. A significant proportion of IMTs show nuclear MDM2 expression[12,15,18].
IMTs are characterized by the presence of receptor tyrosine kinase gene rearrangements. This finding provides further support for the neoplastic nature of IMTs and their differentiation from inflammatory pseudotumors[5,8,12,23,25]. About 50% of IMTs, particularly those arising in young patients, show chromosomal translocations involving the ALK locus on chromosome 2p23, leading to activation of the ALK tyrosine kinase, resulting in ALK protein expression on IHC[5,8,12,19,23,24,26]. Multiple fusion partners to ALK have been described in IMTs, including TPM3, TPM4, CARS, ATIC, SEC31L1, CLTC, among others[5,8,23,25,27-32]. EML4::ALK gene fusions, as present in our case, have been described in IMTs, mostly occurrng in the lung and soft tissue[8]. ALK overexpression can be detected on IHC, however localization within the cells seems to be determined by the fusion partner. In general, a diffuse cytoplasmatic staining is seen due to the cytoplasmatic localization of the fusion partner of ALK, e.g. TPM3, TPM4, CARS, ATIC and SEC31L1[12,19,30-32]. A granular cytoplasmic staining has been described in IMTs with CLTC as fusion partner, a main structural protein of coated vesicles[12,23,28].
EIMS appear to be characterized by an ALK::RANBP2 or RRPB1::ALK fusion gene transcript[5,12,19,21-23,25]. EIMS with an ALK::RANBP2 fusion show a nuclear membrane pattern staining for ALK, presumably due to the heteroassociation of the fusion protein with normal RANBP2 at the nuclear pore[12,19,21,23,25]. EIMS with an RRPB1::ALK fusion show cytoplasmatic ALK expression with perinuclear accentuation. Lee et al[22] suggested, based on the different morphology, molecular fusion transcript and clinical behavior, that EIMS constitutes a distinct subgroup of IMT that is of higher grade, rather than a transformation of conventional IMT. Since these fusion transcripts have not been reported in conventional IMTs, they assume that these specific ALK fusions are directly responsible for the high proliferative status and distinctive epithelioid morphology of EIMS[22].
The presence of ALK protein expression, detected by IHC, or ALK rearrangement are specific diagnostic markers and are very useful and crucial in the differential diagnosis of IMT. ALK gene rearrangements can be detected by FISH. However, equivocal FISH signal counts are occasionally observed. In the study of Yao et al[33], IMTs with an equivocal pattern of ALK signal count, turned out to be ALK fusion-positive by targeted RNA sequencing, suggesting that a low threshold for ALK FISH signal counts in IMTs might be proposed, and that more attention should be paid to equivocal (i.e. split signals in around 15% of counted tumor cells) ALK FISH signal cases. This was also seen in our case with an equivocal signal count (split signals in 12% of counted tumor cells) on FISH, but with a confirmed ALK gene fusion by using targeted RNA NGS. In addition, also ALK positivity on IHC should be interpreted with caution due to the possibility of non-rearrangement-induced ALK protein expression, as seen, for example in spindle cell and alveolar rhabdomyosarcoma. In these cases, amplification or upregulation of ALK may underly immunohistochemical expression of ALK[34-38]. Further, ALK immunoexpression can be negative in ALK-fusion positive IMTs, therefore FISH testing should be performed in IMTs with typical morphologic features, but negative ALK immunostaining[8]. Since only 50% of the IMTs show an ALK rearrangement, the absence of ALK on IHC does not exclude the diagnosis of IMT[12]. ALK-negative IMTs are more common in elderly patients and may show more nuclear atypia or atypical mitoses[15]. For tumors resembling IMTs, but that occur in elderly patients and in unusual anatomical locations, or that demonstrate prominent nuclear atypia, more aggressive spindle cell sarcomas should be included in the differential diagnosis e.g. myofibroblastic sarcoma, leiomyosarcoma, follicular dendritic cell sarcoma, dedifferentiated liposarcoma, ...[12,39]. In contrast, tumors with typical cytoarchitectural features occurring in the lung or abdomen of paediatric and adolescent patients can be diagnosed as IMTs, even without ALK expression[15].
In addition, ROS1 rearrangements were identified in a subset of ALK-negative IMTs, indicating a new diagnostic marker[8,39]. Antonescu et al[8] showed that cytoplasmic ROS1 expression is limited to tumors with ROS1 rearrangements and that ROS1 IHC is consistently negative in ALK-positive IMTs[39]. Also, gene fusions involving NTRK3, PDGFRB, and RET have been reported[40-42]. TP53 mutation is an infrequent event in IMT and may not play a major role in its pathogenesis[18].
Since IMT shows an atypical clinical presentation and nonspecific radiological features, the final diagnosis is made on histology. The WHO’s 2020 essential diagnostic criteria for IMT in the digestive system are as follows: loose fascicles of plump spindle cells without substantial pleomorphism (except epithelioid type); an inflammatory infiltrate of lymphocytes and plasma cells together with SMA positivity and often combined with ALK or (rarely) ROS1 expression[5].
IMT is a neoplasm of intermediate biologic potential with a tendency for local recurrence and persistent local growth. The risk for distant metastasis is small[5,12,13,15,19]. The most common sites of metastasis are lung and brain, followed by liver and bone. Metastatic disease is usually identified at presentation or within a year of diagnosis[12,43]. Coffin et al[15] showed that ALK positivity is associated with local recurrence, but not distant metastasis, which was confined to ALK-negative lesions. Thus, ALK positivity may be a favorable prognostic indicator in IMT. EIMS is more aggressive and recurs rapidly, with disseminated intra-abdominal disease, variable liver metastases, and a high mortality rate[5,19].
The presence of receptor tyrosine kinase gene rearrangements defines therapeutic targets for IMTs, which may respond to tyrosine kinase inhibitors, such as crizotinib with symptomatic improvement, as well as radiologic response[33,34]. Therefore, it is recommended to perform immunohistochemical staining, FISH or NGS to detect an underlying receptor tyrosine kinase gene rearrangement, especially in recurrent/advanced lesions in which systemic therapy with kinase inhibitors could be beneficial[8].
Inflammatory myofibroblastic tumor of the intrapancreatic biliary tract is rare and often difficult to diagnose. In this manuscript, we give a recent update of the clinicopathologic features, focusing on the pathologic and molecular features.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Chan C, Australia; Ikura Y, Japan; Kitamura K, Japan; Lim SC, South Korea; van Leeuwen DJ, United States S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Brunn H. Two interesting benign lung tumors of contradictory histopathology; remarks on necessity for maintaining chest tumor registry. J Thorac Surg. 1939;9:199. [DOI] [Cited in This Article: ] |
| 2. | Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 250] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Lim K, Cho J, Pak MG, Kwon H. Inflammatory Myofibroblastic Tumor of the Pancreas: A Case Report and Literature Review. Taehan Yongsang Uihakhoe Chi. 2020;81:1497-1503. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 4. | Liu Z, Li G, Gou A, Xiao Z, Xu Y, Song S, Guo K, Ma G. Inflammatory myofibroblastic tumor in the pancreatic neck: a rare case report and literature review. Gland Surg. 2021;10:1832-1839. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | WHO classification of tumours (5th edition): Digestive System Tumours. International agency for research on cancer, 2020. [Cited in This Article: ] |
| 6. | Strainiene S, Sedleckaite K, Jarasunas J, Savlan I, Stanaitis J, Stundiene I, Strainys T, Liakina V, Valantinas J. Complicated course of biliary inflammatory myofibroblastic tumor mimicking hilar cholangiocarcinoma: A case report and literature review. World J Clin Cases. 2021;9:6155-6169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Meis-Kindblom JM, Kjellström C, Kindblom LG. Inflammatory fibrosarcoma: update, reappraisal, and perspective on its place in the spectrum of inflammatory myofibroblastic tumors. Semin Diagn Pathol. 1998;15:133-143. [PubMed] [Cited in This Article: ] |
| 8. | Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, Al-Ahmadie H, Fletcher CD, Alaggio R. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 9. | Matsubayashi H, Uesaka K, Sasaki K, Shimada S, Takada K, Ishiwatari H, Ono H. A Pancreatic Inflammatory Myofibroblastic Tumor with Spontaneous Remission: A Case Report with a Literature Review. Diagnostics (Basel). 2019;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Karnak I, Senocak ME, Ciftci AO, Cağlar M, Bingöl-Koloğlu M, Tanyel FC, Büyükpamukçu N. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg. 2001;36:908-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Pungpapong S, Geiger XJ, Raimondo M. Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case report and review of the literature. JOP. 2004;5:360-367. [PubMed] [Cited in This Article: ] |
| 12. | Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 13. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 980] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 14. | Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 298] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 16. | WHO classification of tumours (5th edition): Soft tissue tumours. International agency for research on cancer, 2020. [Cited in This Article: ] |
| 17. | Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999;12:279-286. [PubMed] [Cited in This Article: ] |
| 18. | Yamamoto H, Oda Y, Saito T, Sakamoto A, Miyajima K, Tamiya S, Tsuneyoshi M. p53 Mutation and MDM2 amplification in inflammatory myofibroblastic tumours. Histopathology. 2003;42:431-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Mariño-Enríquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM, Hornick JL. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Donner LR, Trompler RA, White RR 4th. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol. 1996;27:1095-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Chen ST, Lee JC. An inflammatory myofibroblastic tumor in liver with ALK and RANBP2 gene rearrangement: combination of distinct morphologic, immunohistochemical, and genetic features. Hum Pathol. 2008;39:1854-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lee JC, Li CF, Huang HY, Zhu MJ, Mariño-Enríquez A, Lee CT, Ou WB, Hornick JL, Fletcher JA. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017;241:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 422] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 24. | Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 392] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 25. | Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, Zuppan C, Bridge JA. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776-2780. [PubMed] [Cited in This Article: ] |
| 27. | Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CD, Fletcher JA. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001;159:411-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B, De Wolf-Peeters C, Pauwels P, Hagemeijer A, Marynen P. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2002;34:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Debelenko LV, Arthur DC, Pack SD, Helman LJ, Schrump DS, Tsokos M. Identification of CARS-ALK fusion in primary and metastatic lesions of an inflammatory myofibroblastic tumor. Lab Invest. 2003;83:1255-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Debiec-Rychter M, Marynen P, Hagemeijer A, Pauwels P. ALK-ATIC fusion in urinary bladder inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;38:187-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Panagopoulos I, Nilsson T, Domanski HA, Isaksson M, Lindblom P, Mertens F, Mandahl N. Fusion of the SEC31L1 and ALK genes in an inflammatory myofibroblastic tumor. Int J Cancer. 2006;118:1181-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Yao Q, Bai Q, Zhang X, Ji G, Chang H, Cai X, Yu L, Wang J, Zhu X, Zhou X. Assessment of ALK Fusions in Uncommon Inflammatory Myofibroblastic Tumors With ALK IHC Positivity but FISH-Equivocal Findings by Targeted RNA Sequencing. Arch Pathol Lab Med. 2022;146:1234-1242. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 34. | Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, Kwak EL, Clark JW, Wilner KD, Christensen JG, Jänne PA, Maki RG, Demetri GD, Shapiro GI. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727-1733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 35. | Chebib I, Taylor MS, Nardi V, Rivera MN, Lennerz JK, Cote GM, Choy E, Lozano Calderón SA, Raskin KA, Schwab JH, Mullen JT, Chen YE, Hung YP, Nielsen GP, Deshpande V. Clinical Utility of Anchored Multiplex Solid Fusion Assay for Diagnosis of Bone and Soft Tissue Tumors. Am J Surg Pathol. 2021;45:1127-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2910] [Cited by in F6Publishing: 2829] [Article Influence: 257.2] [Reference Citation Analysis (0)] |
| 37. | Hayes DF. Considerations for Implementation of Cancer Molecular Diagnostics Into Clinical Care. Am Soc Clin Oncol Educ Book. 2016;35:292-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Le Loarer F, Cleven AHG, Bouvier C, Castex MP, Romagosa C, Moreau A, Salas S, Bonhomme B, Gomez-Brouchet A, Laurent C, Le Guellec S, Audard V, Giraud A, Ramos-Oliver I, Cleton-Jansen AM, Savci-Heijink DC, Kroon HM, Baud J, Pissaloux D, Pierron G, Sherwood A, Coindre JM, Bovée JVMG, Larousserie F, Tirode F. A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol. 2020;33:404-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 39. | Hornick JL, Sholl LM, Dal Cin P, Childress MA, Lovly CM. Expression of ROS1 predicts ROS1 gene rearrangement in inflammatory myofibroblastic tumors. Mod Pathol. 2015;28:732-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA, Coffin CM. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 41. | Yamamoto H, Yoshida A, Taguchi K, Kohashi K, Hatanaka Y, Yamashita A, Mori D, Oda Y. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology. 2016;69:72-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 42. | Alassiri AH, Ali RH, Shen Y, Lum A, Strahlendorf C, Deyell R, Rassekh R, Sorensen PH, Laskin J, Marra M, Yip S, Lee CH, Ng TL. ETV6-NTRK3 Is Expressed in a Subset of ALK-Negative Inflammatory Myofibroblastic Tumors. Am J Surg Pathol. 2016;40:1051-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Morotti RA, Legman MD, Kerkar N, Pawel BR, Sanger WG, Coffin CM. Pediatric inflammatory myofibroblastic tumor with late metastasis to the lung: case report and review of the literature. Pediatr Dev Pathol. 2005;8:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |