Copyright
©The Author(s) 2023.
World J Clin Cases. May 26, 2023; 11(15): 3542-3551
Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3542
Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3542
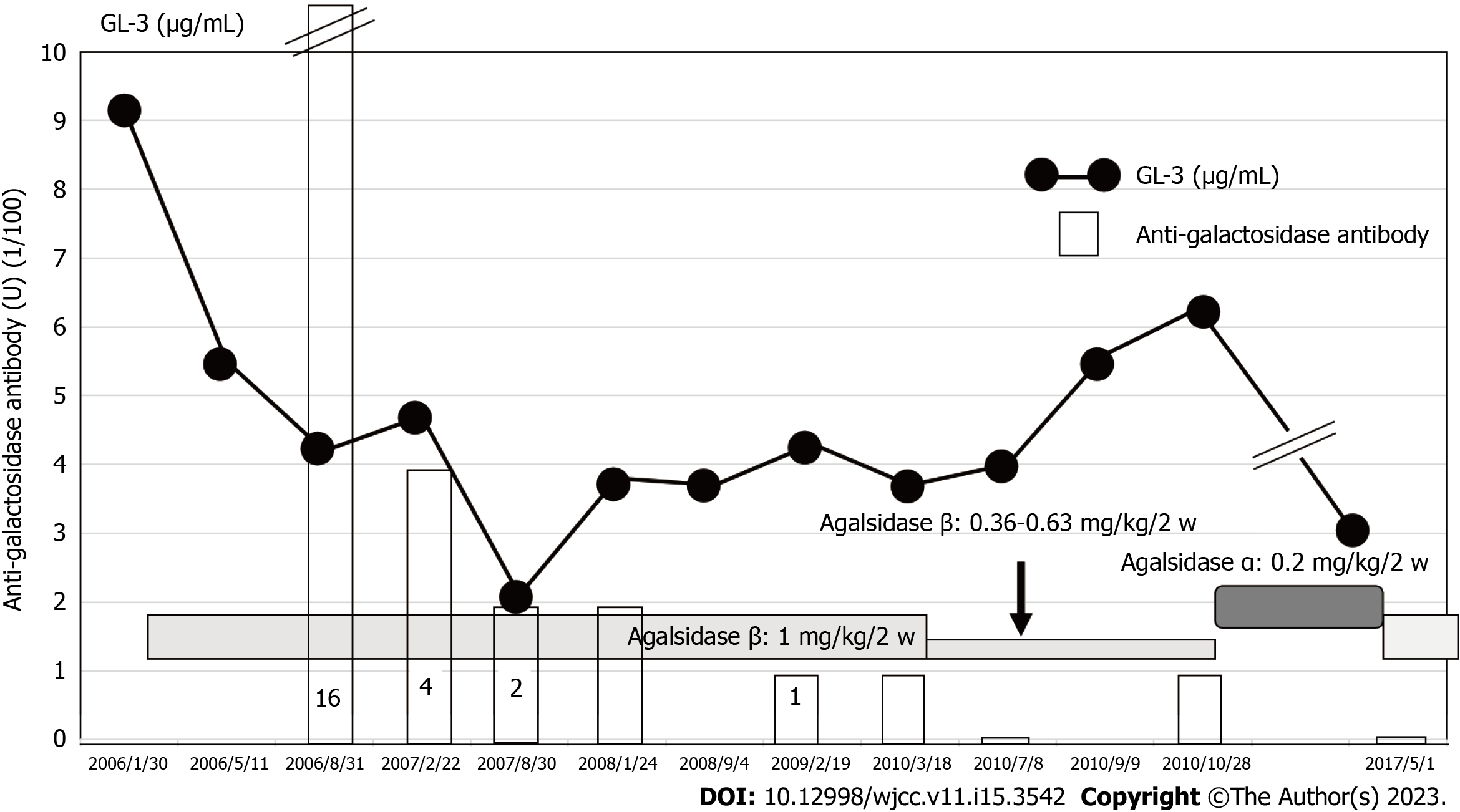
Figure 7 Effects of agalsidase β administration on globotriaosylceramide and anti-galactosidase antibody levels in Case 2.
Globotriaosylceramide (GL-3) was sufficiently reduced when 1 mg/kg of agalsidase β was administered; GL-3 increased as agalsidase β dose decreased. α-galactosidase at a dose of 0.2 mg/kg, initiated from 2010 and replaced by agalsidase β in 2017, suppressed GL-3 level within normal limits. GL-3: Globotriaosylceramide.
- Citation: Harigane Y, Morimoto I, Suzuki O, Temmoku J, Sakamoto T, Nakamura K, Machii K, Miyata M. Enzyme replacement therapy in two patients with classic Fabry disease from the same family tree: Two case reports. World J Clin Cases 2023; 11(15): 3542-3551
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3542