Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.1
Peer-review started: September 26, 2022
First decision: October 18, 2022
Revised: October 29, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 6, 2023
The central nervous system (CNS) is a reservoir of immune privilege. Specialized immune glial cells are responsible for maintenance and defense against foreign invaders. The blood–brain barrier (BBB) prevents detrimental pathogens and potentially overreactive immune cells from entering the periphery. When the double-edged neuroinflammatory response is overloaded, it no longer has the protective function of promoting neuroregeneration. Notably, microbiota and its derivatives may emerge as pathogen-associated molecular patterns of brain pathology, causing microbiome–gut–brain axis dysregulation from the bottom-up. When dysbiosis of the gastrointestinal flora leads to subsequent alterations in BBB permeability, peripheral immune cells are recruited to the brain. This results in amplification of neuroinflammatory circuits in the brain, which eventually leads to specific neurological disorders. Aggressive treatment strategies for gastro
Core Tip: Neurological disorders are increasingly diagnosed globally owing to the disruption of the gut-brain axis. The impact of dysbiosis on the gut microbiota often plays a crucial role in disease pathogenesis. A thorough understanding of this complex relationship is essential for the development of new management strategies against various neurological disorders.
- Citation: Lin MS, Wang YC, Chen WJ, Kung WM. Impact of gut–brain interaction in emerging neurological disorders. World J Clin Cases 2023; 11(1): 1-6
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.1
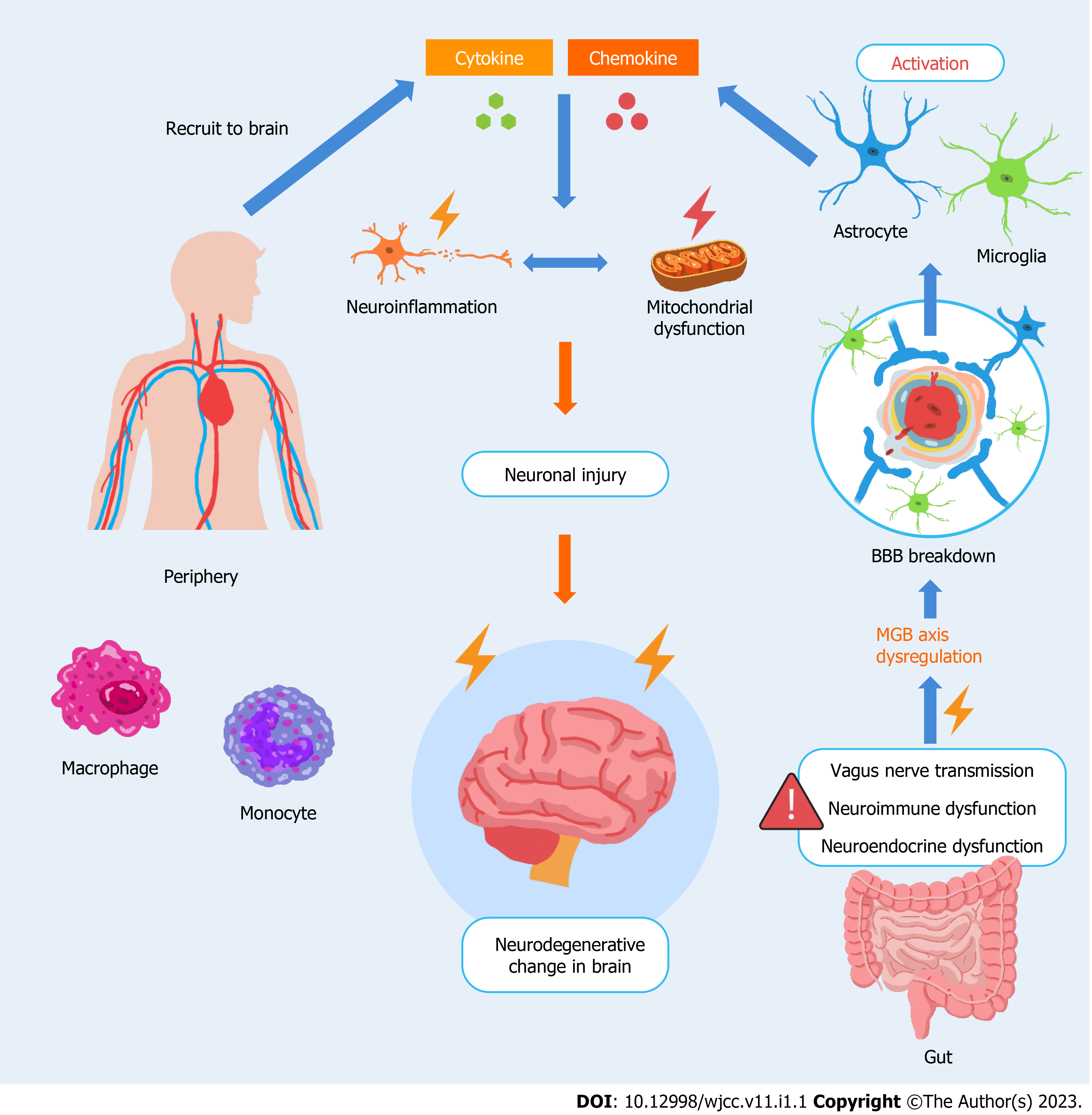
An increasing number of preclinical and clinical studies have provided evidence on how various neurological disorders result from an imbalance of the gut–brain interaction. A recent animal model study reported that gut-derived metabolites can substantially influence mouse behaviors[1]. Moreover, a multicenter randomized controlled trial performed by Korean investigators concluded that probiotics may help improve cognitive dysfunction in older adults[2]. In this editorial, we summarize and demonstrate the mechanisms of the complex gut–brain interaction in neurological disorders (Figure 1), providing a pivotal solution for scientists, researchers, and clinicians to protect the brain. Refined treatment schemes for gut disorders and related microbiota environments may be beneficial in improving the prognosis of neurological disorders such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and diabetic neuropathy[3,4]. Considering the growing body of relevant literature, aggressive therapeutic strategies for gastrointestinal disorders may be implicated to protect the peculiar immune responses from the gastrointestinal disequilibrium that causes specific diseases of the nervous system. We believe that the gut–brain axis and balanced microbiota play a considerable role in a diverse spectrum of neurological disorders and can serve as a basis for future investigations.
In the central nervous system (CNS), astrocytes, which are dedicated glial cells, are the majority, exceeding the number of neurons by a factor of five[5]. In embryology studies, astrocytes, similar to neurons, emanate from neuroepithelial precursors[6]. The presence of astrocytes in the CNS plays a critical role in its overall maintenance and homeostasis. The cardinal features of astrocytes include the buffering of potassium across the CNS, elimination and retrieval of glutamate, and maintenance of water equilibrium and osmotic pressure of the microenvironment. Furthermore, astrocytes are innate immune cells that can mediate neuroinflammatory responses in the CNS[7] and generate neurotrophins, such as brain-derived neurotrophic factors, and anti-inflammatory cytokines, such as interleukin 10[8].
Microglia are innate immune cells of the CNS that are embryologically derived from myeloid progenitor cells and are homologous to the macrophage series in the peripheral blood[9]. With their homology to macrophages, microglia function as immune pioneers in the CNS by promoting immune responses to stabilize and homogenize the microenvironment of the brain. Microglial activation may occur as an initial step in the neuroinflammatory response due to dysregulation of immune regulation in age-related neural diseases or abnormal folding/aggregation of proteins resulting from environmental or genetic factors. Stimulated microglia may trigger a more intense neuroinflammatory reaction via the reactivation of astrocytes. Through the microglia–astrocyte crosstalk, these two series of glial-derived innate immune cells may mutually modulate the innate immune defense system of the CNS[10,11].
In combination with microvascular endothelial cells, pericytes, and basement membranes, the endfeet of astrocytes encircle the capillaries, resulting in an almost completely gapless blood-brain-barrier (BBB) via gap junctions. While the BBB allows the passage of a minor fraction of lipids or molecules with molecular weights less than 400 Da[12], it can prevent external substances, bacteria, or viruses from entering the brain through the peripheral blood circulation.
With an excessive neuroinflammatory response to stress in the brain, the activation of astrocytes and subsequent reactive astrogliosis may contribute to alterations in BBB permeability[13]. Accordingly, owing to the compromise of this defense line between the peripheral system and brain, several proinflammatory cytokines and chemokines, which are released owing to glial activation, recruit peripheral innate immune cells, such as neutrophils, monocytes, macrophages, natural killer cells, and dendritic cells, to shift toward the brain[14]. During neuroinflammation in the CNS, innate and peripheral immune cells are excessively augmented, and glial cytokines/chemokines amplify the inflammatory signal, resulting in a vicious cycle. The neuroinflammatory response amplifies and progresses to mitochondrial dysfunction in the principal neurons or surrounding cells of the CNS, eventually leading to degenerative brain damage[15]. The pathological state of BBB permeability is involved in brain inflammation, resulting from the alternation of gut microbiota or lipopolysaccharides released from the intestine into the bloodstream[16]. This underlies the microbiome-gut-brain (MGB) axis and provides a rationale for the close relationship between an unhealthy gut and brain diseases. Dysbiosis, an abnormal composition in microbiota, may be caused by conditions such as aging[17], gastrointestinal diseases[18], and renal transplantation[19,20].
In general, progressing from the bottom-up, the gut may interact with the brain via three main pathways. The vagus nerve innervates the brainstem and gut via a direct conduit. Lactobacillus rhamnosus (JB-1) affects the γ-aminobutyric acid (GABA) receptors in brain regions associated with mood and anxiety[21]. The aggregation of α-synuclein (Lewy bodies) disseminates from the enteric nervous system to the CNS, exacerbating the symptoms of patients with Parkinson’s disease[22], since vagotomy eliminates the extent of pathogenesis. Predisposing factors that precipitate increased intestinal permeability, such as gastrointestinal inflammation or infection, may contribute to systemic inflammation caused by pathological antigens or dietary allergens. These pathogen-associated molecular patterns or proinflammatory cytokines may circulate toward the brain, causing subsequent cerebral inflammation. For example, short-chain fatty acids, which are microbial-derived intermediates, can penetrate the BBB, react with microglia, and subsequently trigger neuroinflammation in the brain[23]. Increased permeability of the intestinal barrier, resulting from high-fat consumption and long-term inflammation of the gastrointestinal tract[24], may lead to the distribution of lipopolysaccharides from the gut into the circulation, stimulating systemic inflammatory activation and eventual destruction of the BBB[25], along with inflammation-driven neurodegeneration in the brain. Furthermore, the metabolites produced by intestinal microbiota, analogous to hormones, affect the functioning of the neuroendocrine system in the brain via the systemic circulation, serving as the bottom-up signaling pathway involved in the MGB axis. For example, GABA, derived from Levilactobacillus brevis and Bifidobacterium dentium among the gut microbiota, can traverse the BBB[26] and may affect neurotransmission homeostasis, ultimately leading to neurodegenerative diseases.
The CNS, which is an immune-privileged site, maintains the equilibrium of the system through a supportive and defensive glial cell cohort that elicits neuroinflammatory responses. However, neuroinflammation, which is a double-edged sword, can be confronted by a load level that exceeds its protective capacity. The stress signals originating from the periphery likely interfere with the CNS, rendering neuroinflammation in the brain detrimental and disrupting the homeostasis of the neuroendocrine system. These warning signals from the gastrointestinal tract cause the dysregulation of the MGB axis. Thus, the future development of medical and molecular therapies must target the molecular focus from the gut to the brain.
The authors would like to thank Mr. Feng-Ming Hsu for drawing Figure 1.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Salvadori M, Italy; Wen XL, China S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu WL, Rabut C, Ladinsky MS, Hwang SJ, Guo Y, Zhu Q, Griffiths JA, Knight R, Bjorkman PJ, Shapiro MG, Geschwind DH, Holschneider DP, Fischbach MA, Mazmanian SK. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 2. | Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, Shin DM. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J Gerontol A Biol Sci Med Sci. 2021;76:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 3. | Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, McCormick B, Sampson TR, Alam A, Ye K. Gut microbiota regulate Alzheimer's disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71:2233-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 4. | Oroojzadeh P, Bostanabad SY, Lotfi H. Psychobiotics: the Influence of Gut Microbiota on the Gut-Brain Axis in Neurological Disorders. J Mol Neurosci. 2022;72:1952-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 5. | Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3031] [Cited by in F6Publishing: 3411] [Article Influence: 243.6] [Reference Citation Analysis (0)] |
| 6. | Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 697] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 7. | Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275 Pt 3:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 488] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 8. | Szpakowski P, Ksiazek-Winiarek D, Turniak-Kusy M, Pacan I, Glabinski A. Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli. Biomedicines. 2022;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Zhang J, You Z. Switching of the Microglial Activation Phenotype Is a Possible Treatment for Depression Disorder. Front Cell Neurosci. 2018;12:306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 11. | Jha MK, Jo M, Kim JH, Suk K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist. 2019;25:227-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 325] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 12. | Dong X. Current Strategies for Brain Drug Delivery. Theranostics. 2018;8:1481-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 495] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 13. | Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta. 2016;1862:483-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 14. | Kip E, Parr-Brownlie LC. Reducing neuroinflammation via therapeutic compounds and lifestyle to prevent or delay progression of Parkinson's disease. Ageing Res Rev. 2022;78:101618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Kung WM, Lin MS. The NFκB Antagonist CDGSH Iron-Sulfur Domain 2 Is a Promising Target for the Treatment of Neurodegenerative Diseases. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Deidda G, Biazzo M. Gut and Brain: Investigating Physiological and Pathological Interactions Between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases. Front Neurosci. 2021;15:753915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Salazar N, González S, Nogacka AM, Rios-Covián D, Arboleya S, Gueimonde M, Reyes-Gavilán CGL. Microbiome: Effects of Ageing and Diet. Curr Issues Mol Biol. 2020;36:33-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Trakman GL, Fehily S, Basnayake C, Hamilton AL, Russell E, Wilson-O'Brien A, Kamm MA. Diet and gut microbiome in gastrointestinal disease. J Gastroenterol Hepatol. 2022;37:237-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ardalan M, Vahed SZ. Gut microbiota and renal transplant outcome. Biomed Pharmacother. 2017;90:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Salvadori M, Tsalouchos A. Microbiota, renal disease and renal transplantation. World J Transplant. 2021;11:16-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 21. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050-16055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2500] [Cited by in F6Publishing: 2309] [Article Influence: 177.6] [Reference Citation Analysis (0)] |
| 22. | Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 538] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 23. | Wenzel TJ, Gates EJ, Ranger AL, Klegeris A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci. 2020;105:103493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 2022;36:e24420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Osadchiy V, Martin CR, Mayer EA. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 2019;17:322-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 26. | Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 665] [Article Influence: 55.4] [Reference Citation Analysis (2)] |