Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11590
Peer-review started: June 30, 2022
First decision: August 1, 2022
Revised: August 17, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: November 6, 2022
Primary hepatic angiosarcoma (PHA) is a rare malignant tumor of the vascular endothelium. Clinical manifestations and laboratory and imaging examinations often lack specificity for PHA. We report a case of PHA, and describe the ultra
A 75-year-old woman presented with right upper quadrant abdominal pain for half a month. Magnetic resonance imaging (MRI) at a local hospital revealed multiple liver space-occupying lesions, and she was admitted to our hospital for further diagnosis. Contrast-enhanced ultrasound (CEUS) revealed multiple slightly hyperechoic nodules in the liver, which were suspected to be of malignant vascular origin. Contrast-enhanced computed tomography revealed multiple low-density nodules in the liver, considered to be metastatic hematopoietic malignancies. Contrast-enhanced MRI showed that the multiple liver nodules shared features with infectious lesions. Laboratory examination revealed normal alpha-fetoprotein levels, slightly increased other liver enzymes, decreased platelets, and significantly increased D-dimer levels. Liver biopsy and histopathology confirmed the presence of PHA.
CEUS can provide valuable clues for the diagnosis of PHA and greatly improve the success rate of puncture biopsy.
Core Tip: In this study, we showed a rare reported disease named primary hepatic angiosarcoma (PHA). In this case report, we focused on diagnosing PHA by contrast-enhanced ultrasound (CEUS). Meanwhile, we introduced a new ultrasound technology, and CEUS has many specific signs in the diagnosis and differential diagnosis of PHA. It has great advantages in displaying microperfusion, microvessels and necrosis of PHA and has great clinical value in diagnosing PHA. Our findings regarding CEUS contribute to the more accurate and earlier diagnosis of PHA and provide a longer survival time in the future.
- Citation: Wang J, Sun LT. Primary hepatic angiosarcoma: A case report. World J Clin Cases 2022; 10(31): 11590-11596
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11590.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11590
Primary hepatic angiosarcoma (PHA) is an invasive malignant stromal tumor that is extremely rare, accounting for approximately 2% of primary liver tumors. Studies have proposed that PHA is related to chemical pollution[1]. The clinical manifestations and laboratory tests for PHA lack specificity; therefore, imaging examinations are important for diagnosing PHA. Current studies have focused on the description of computed tomography (CT) and magnetic resonance imaging (MRI) of PHA; however, reports on ultrasound, especially contrast-enhanced ultrasound (CEUS), are still limited[2,3]. Herein, we report the case of a patient with PHA, rectal cancer, and syphilis.
A 75-year-old woman presented with right upper quadrant abdominal pain.
The patient experienced sudden right upper quadrant abdominal pain for half a month without obvious induction. MRI at the local hospital revealed multiple space-occupying hepatic lesions. The patient was admitted to our hospital for further evaluation.
The patient had a history of hypertension, syphilis (active period and receiving treatment), lower extremity deep venous thrombosis, and pulmonary embolism.
The patient revealed no pertinent personal or family history.
Physical examination revealed marked tenderness in the right upper quadrant of the abdomen and with no obvious abnormalities during the remainder of the examination.
Tumor biomarkers including neuron-specific enolase of 50.4 ng/mL (normal range: 0.0-20.0 ng/mL), human epididymal protein of 174.3 pmol/L (normal range: 0.0-121.0 pmol/L), carbohydrate antigen 125 of 139.4 U/mL (normal range: 0.0-35.0 U/mL), gastrin-releasing peptide precursor of 109.2 pg/mL (normal range: 25.0-78.0 pg/mL). The D-dimer level was 31200 μg/L (normal range: 0.0-550.0 μg/L), and the platelet count was 39 × 109/L (normal range: 125.0-350.0 × 109/L).
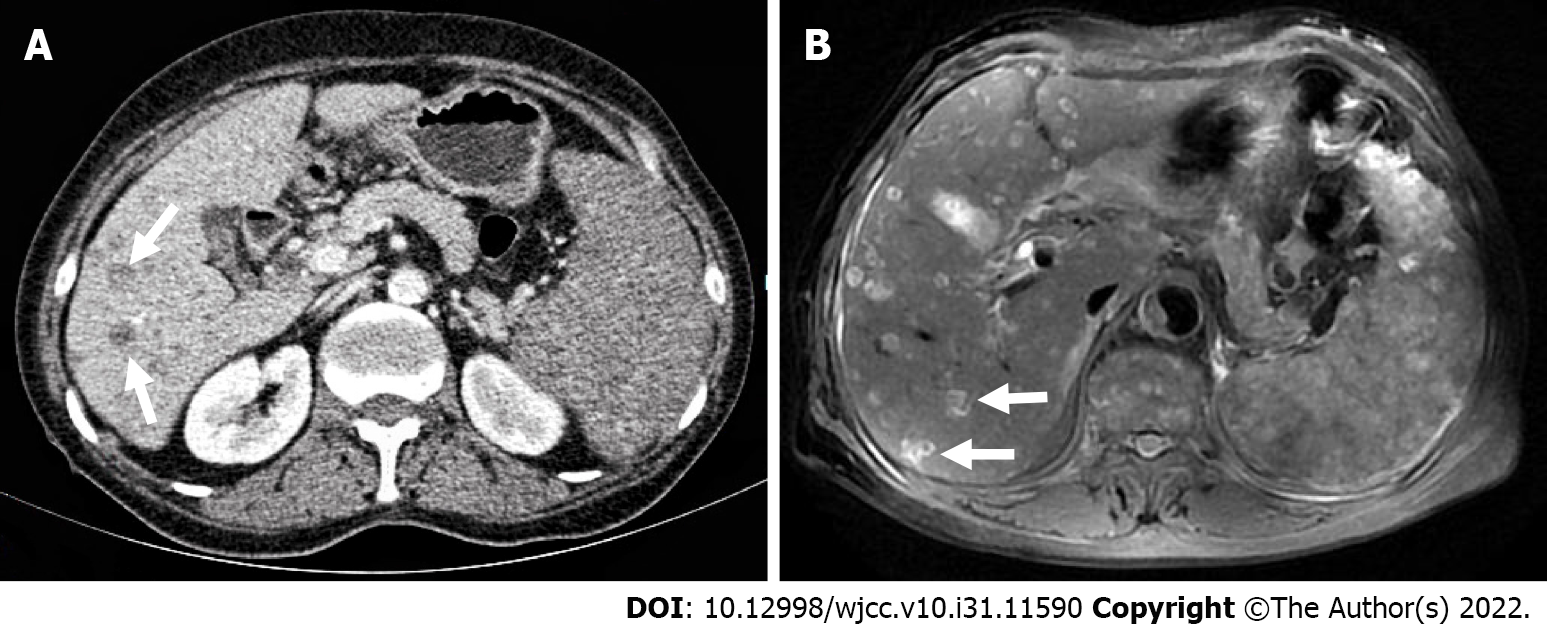
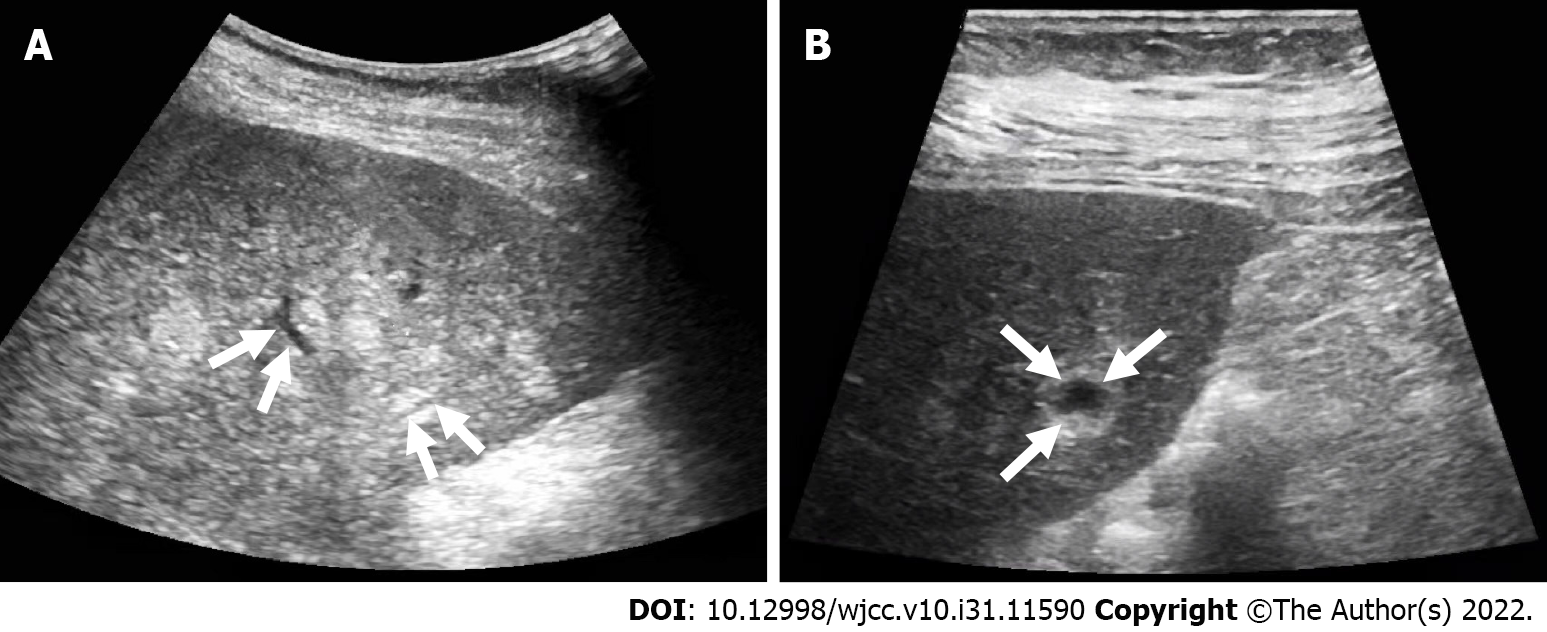
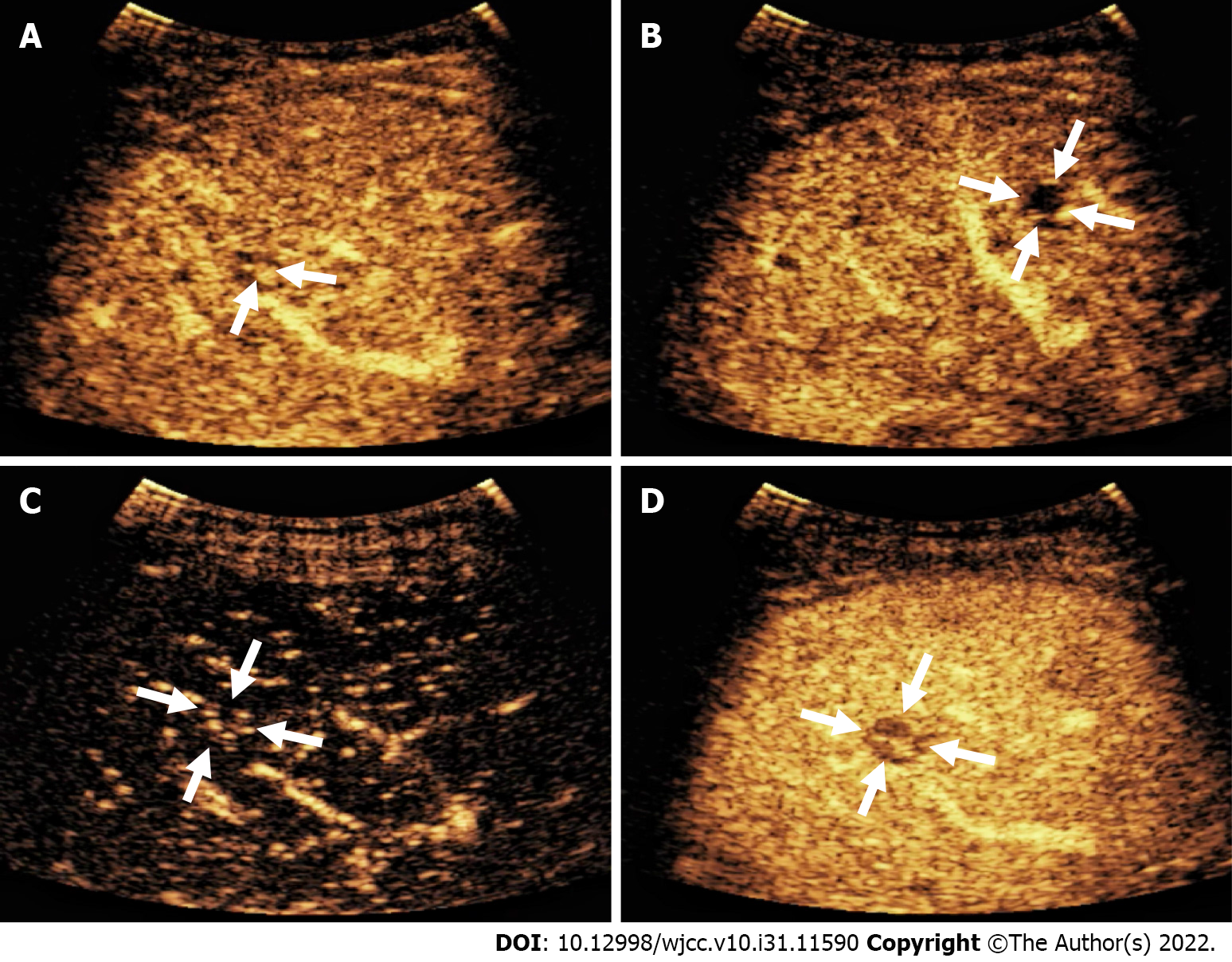
The patient underwent contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI) at our hospital, which revealed multiple low-density nodules with mild enhancement in the liver, considered to have metastasized from hematopoietic malignancies; moreover, CEMRI suggested infectious lesions (Figure 1). Conventional ultrasound revealed multiple slightly hyperechoic nodules with unclear boundaries and loose inner structures in the liver; some exhibited vascular-like structures and anechoic areas; therefore, we suspected angiogenic tumors (Figure 2). CEUS and liver needle biopsy were performed for further diagnosis. CEUS revealed nodular peripheral enhancement in the arterial and portal phases and low enhancement in the late phase. Non-enhanced areas appeared in the nodules, suggesting angiogenic malignant tumors (Figure 3). PHA was pathologically diagnosed based on the needle biopsy results.
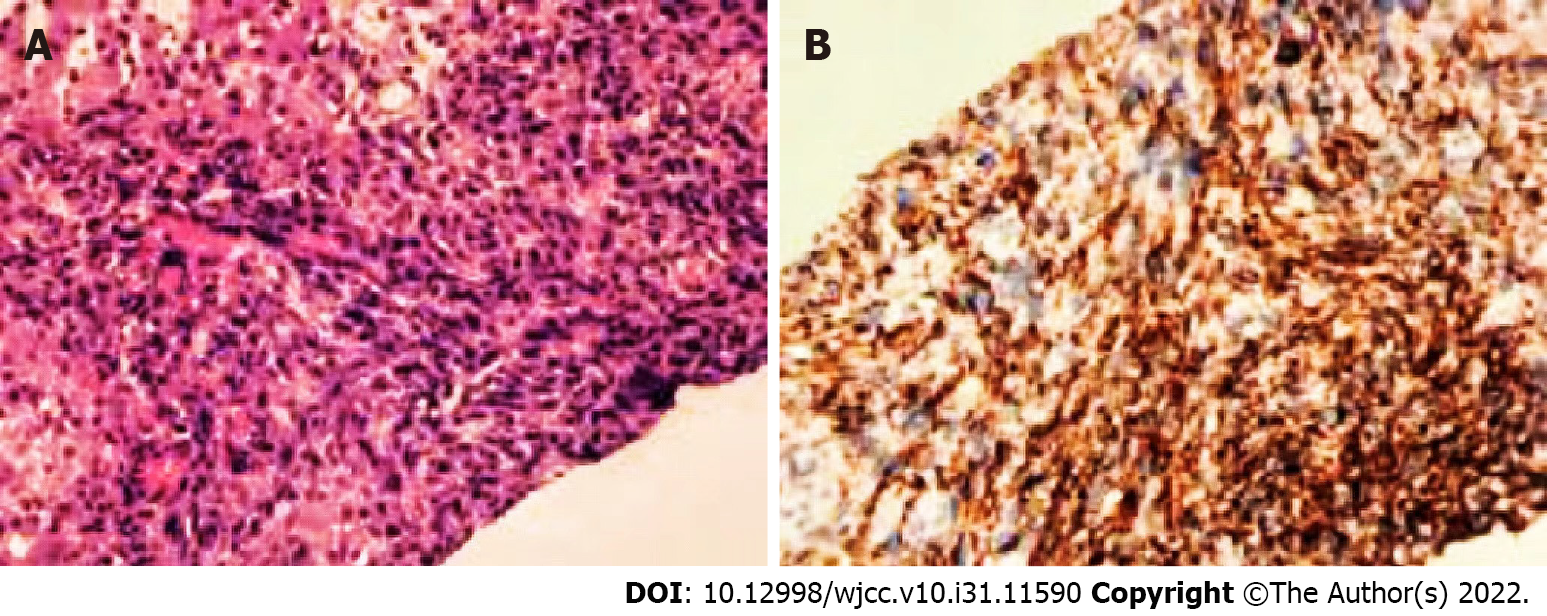
Liver biopsy results confirmed that the nodules were malignant. Immunohistochemistry supported the presence of angiogenesis, indicating angiosarcoma. Immunohistochemical staining revealed the following: CD34 (+), CD31 (+), CK (Pan) (-), CK7 (-), desmin (-), WT (-), and Ki67 (hot spot 60%) (Figure 4).
Due to the multiple metastases and poor body condition, the patient had missed the opportunity to receive the optimal treatment. Next, tislelizumab injection was used as antitumor therapy. Moreover, some measures, including blood transfusions, were used for symptomatic treatment.
After discharge from our hospital, the patient received follow-up evaluations and symptomatic treatment at a local hospital. Ultimately, the patient died of septic shock due to poor physical condition.
PHA has a low incidence rate, occult onset, and an unknown etiology. Studies have indicated that 25% of PHA are related to exposure to vinyl chloride, thorium oxide, arsenic, and radium; however, most are idiopathic. PHA is associated with high invasiveness, easy recurrence and metastasis, poor prognosis, and a short survival time, generally between 6 and 16 mo[4]. According to morphology, it may present as large masses, diffuse nodules, and multiple nodules[5].
As the disease onset is insidious, symptoms such as pain, weakness, fatigue, and weight loss are usually caused by the secondary effects of the tumor. To date, PHA lacks specific tumor markers. Studies have reported that PHA is closely related to D-dimer and platelet levels, manifesting as increased D-dimer levels and thrombocytopenia. Physiological conditions caused by malignant endodermic cells can promote platelet adhesion and activation, leading to the excessive consumption of platelets and coagulation factors at the tumor site[6,7].
Imaging is an effective method for preliminary diagnosis. Conventional ultrasound examination of this case suggested angiogenic tumors; however, it is difficult to distinguish between hemangioma, metastasis, and hepatocellular carcinomas. In particular, it seems more difficult to distinguish it from metastasis from an earlier rectal cancer. The patient underwent CEUS and CECT, as well as CEMRI for further diagnosis. CEUS revealed nodular peripheral enhancement in the arterial and portal phases and low enhancement in the late phase, accompanied by non-enhanced areas in the nodules. These non-enhanced areas were filled with hemorrhagic, necrotic, and fibrous components, resulting in the non-enhanced signs[8]. This case differed from the angiography mode of hemangioma, metastasis, and hepatocellular carcinomas. CEUS of the hemangioma revealed nodular hyperenhancement in the arterial phase, which continued into the delayed phase. Among metastatic tumors, ring enhancement of nodules can be observed in the arterial phase, which begins to subside in the portal phase. The characteristics of CEUS in hepatocellular carcinoma include high enhancement in the arterial phase and clearance in the late arterial phase or early portal phase (wash-in and wash-out)[9]. In the present case, CEUS imaging revealed completely different signs from the typical signs of hemangioma, metastasis, and liver cancer. According to the literature, CEUS has high diagnostic value for PHA[10]. In brief, when tumors exhibit nodular peripheral enhancement in the arterial and portal phases and low enhancement in the late phase, some may be accompanied by non-enhanced areas, and PHA should be considered[10,11]. However, CECT imaging revealed features typically associated with hematological malignancies, while CEMRI suggested infectious lesions. Therefore, liver biopsy is necessary for a definitive diagnosis. Koyama et al[12] reported a 78% success rate for biopsy, which was due to the high probability of necrosis and bleeding in the tumor. CEUS can identify necrotic and hemorrhagic areas, and reduce false-negative results from biopsy. Finally, a CEUS-mediated biopsy was performed to clarify the nature of the nodules.
Pathologically, PHA has highly disordered vascular characteristics and heterogeneity; therefore, 80% of PHA show heterogeneous branches and scaffold-like vascular structures, distinguishing them from hepatic hemangioma[13,14]. The present study showed that PHA expresses at least one among CD31-, CD34-, and factor 8-related antigens and that the Ki-67 value-added index is > 10%, which is a common diagnostic feature of angiosarcoma[15,16]. In this study, CD34 (+), CD31 (+), and Ki67 (hot spot 60%) expression was consistent with that reported in the literature.
Due to the low incidence rate of PHA, no clear treatment guidelines currently exist. The main therapeutic strategy for PHA is surgery, supplemented by radiotherapy and chemotherapy[17,18]. Surgery is considered the optimal treatment method, as it provides the best survival outcomes, especially for single tumors with a diameter of < 10 cm[19,20]. Recent studies have found that targeted therapy has good efficacy in PHA, mainly because the overexpression of VEGF is considered the most important angiogenic factor in different types of sarcomas, including angiosarcoma. However, these few clinical cases need to be confirmed via multicenter studies[21-23].
Owing to the low incidence rate of PHA, its etiology, pathogenesis, and prognosis are unclear, which makes it difficult to diagnose. The presence of thrombocytopenia, D-dimer elevation, and suspicion of PHA on imaging examinations can assist in the diagnosis. CEUS can provide valuable information for diagnosis; however, the diagnosis depends on pathology results. Currently, surgery is the optimal treatment, and targeted therapy may become a promising approach in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Aydin S, Turkey; Liu X, China; Wang P, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol. 2015;41:1137-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Pickhardt PJ, Kitchin D, Lubner MG, Ganeshan DM, Bhalla S, Covey AM. Primary hepatic angiosarcoma: multi-institutional comprehensive cancer centre review of multiphasic CT and MR imaging in 35 patients. Eur Radiol. 2015;25:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Ling W, Qiu T, Ma L, Lei C, Luo Y. Contrast-enhanced ultrasound in diagnosis of primary hepatic angiosarcoma. J Med Ultrason (2001). 2017;44:267-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Huang NC, Kuo YC, Chiang JC, Hung SY, Wang HM, Hung YM, Chang YT, Wann SR, Chang HT, Wang JS, Ho SY, Guo HR. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore). 2015;94:e816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Semelka RC, Nimojan N, Chandana S, Ramalho M, Palmer SL, DeMulder D, Parada Villavicencio C, Woosley J, Garon BL, Jha RC, Miller FH, Altun E. MRI features of primary rare malignancies of the liver: A report from four university centres. Eur Radiol. 2018;28:1529-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Wadhwa S, Kim TH, Lin L, Kanel G, Saito T. Hepatic angiosarcoma with clinical and histological features of Kasabach-Merritt syndrome. World J Gastroenterol. 2017;23:2443-2447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Strainienė S, Jauniškis K, Savlan I, Pamedys J, Stundienė I, Liakina V, Valantinas J. Paraneoplastic Phenomena of Disseminated Intravascular Coagulopathy in Hepatic Angiosarcoma - Rare, Challenging and Fatal. Case Report and Literature Review. Acta Med Litu. 2021;28:330-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Zhou Y, Hou P, Wang F, Li B, Gao J. Primary hepatic malignant vascular tumors: a follow-up study of imaging characteristics and clinicopathological features. Cancer Imaging. 2020;20:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579-2604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 10. | Trojan J, Hammerstingl R, Engels K, Schneider AR, Zeuzem S, Dietrich CF. Contrast-enhanced ultrasound in the diagnosis of malignant mesenchymal liver tumors. J Clin Ultrasound. 2010;38:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Wang L, Lv K, Chang XY, Xia Y, Yang ZY, Jiang YX, Dai Q, Tan L, Li JC. Contrast-enhanced ultrasound study of primary hepatic angiosarcoma: a pitfall of non-enhancement. Eur J Radiol. 2012;81:2054-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology. 2002;222:667-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Kim B, Byun JH, Lee JH, Park BJ, Kwon HJ, Lee SJ, Won HJ, Shin YM, Kim PN. Imaging findings of primary hepatic angiosarcoma on gadoxetate disodium-enhanced liver MRI: comparison with hepatic haemangiomas of similar size. Clin Radiol. 2018;73:244-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Jiang L, Xie L, Li G, Xie H, Fang Z, Cai X, Chen Y. Clinical characteristics and surgical treatments of primary hepatic angiosarcoma. BMC Gastroenterol. 2021;21:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Geller RL, Hookim K, Sullivan HC, Stuart LN, Edgar MA, Reid MD. Cytologic features of angiosarcoma: A review of 26 cases diagnosed on FNA. Cancer Cytopathol. 2016;124:659-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Wang ZB, Wei LX. [Primary hepatic angiosarcoma: a clinical and pathological analysis]. Zhonghua Bing Li Xue Za Zhi. 2013;42:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 17. | Zheng YW, Zhang XW, Zhang JL, Hui ZZ, Du WJ, Li RM, Ren XB. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Kim SJ, Rhu J, Kim JM, Choi GS, Joh JW. Surgical treatment outcomes of primary hepatic sarcomas: A single-center experience. World J Hepatol. 2021;13:584-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Huang IH, Wu YY, Huang TC, Chang WK, Chen JH. Statistics and outlook of primary hepatic angiosarcoma based on clinical stage. Oncol Lett. 2016;11:3218-3222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Li DB, Si XY, Wan T, Zhou YM. A pooled analysis of treatment and prognosis of hepatic angiosarcoma in adults. Hepatobiliary Pancreat Dis Int. 2018;17:198-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Cao J, Wang J, He C, Fang M. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313. [PubMed] [Cited in This Article: ] |
| 22. | Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med. 2012;2:a006593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Linfeng Q, Xingjie X, Henry D, Zhedong W, Hongfei X, Haige Z. Cardiac angiosarcoma: A case report and review of current treatment. Medicine (Baltimore). 2019;98:e18193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |