Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11536
Peer-review started: June 7, 2022
First decision: August 4, 2022
Revised: August 17, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
As an extramedullary form of proliferating myeloblasts, granulocytic sarcoma (GS) is common in patients with acute myeloid leukemia. GS in the central nervous system is rare, and an intraspinal space-occupying lesion caused by GS is even rarer. Surgical decompression is often necessary to remove the intraspinal space-occupying lesion. To the best of our knowledge, we report, for the first time a case of GS that caused extensive compression in the spinal canal without surgical decompression treatment.
A 15-year-old male suddenly developed numbness and weakness in his lower limbs for 10 d, which affected his walking ability. Acute myeloid leukemia was later diagnosed in the Department of Hematology. Magnetic resonance imaging revealed that multiple segmental space-occupying lesions were causing severe spinal cord compression in the thoracic spinal canal. As a result, the patient received routine chemotherapy before surgery. Interestingly, the intraspinal space-occupying lesions completely diminished on magnetic resonance imaging after a course of chemotherapy, and the sensation and strength in his lower limbs markedly recovered.
An intraspinal space-occupying lesion could be the first symptom of acute myeloid leukemia, causing spinal nerve compression without any other symptoms. Following standard chemotherapy, spinal canal compression can be quickly relieved, and the spinal cord and nerve function restored, avoiding emergency surgery.
Core Tip: This case is important as not all intraspinal granulocytic sarcoma in acute myeloid leukemia or chronic myeloid leukemia appear after systemic symptoms. We suggest that if conditions permit, relief of spinal cord compression can be achieved by standard chemotherapy. However, if hematological diseases are excluded, emergency surgery or biopsy is necessary to preserve the spinal cord and nerve function.
- Citation: Shao YD, Wang XH, Sun L, Cui XG. Granulocytic sarcoma with long spinal cord compression: A case report. World J Clin Cases 2022; 10(31): 11536-11541
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11536.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11536
Acute myeloid leukemia (AML) remains the most common acute leukemia in adults, including all non-lymphocytic acute leukemias[1]. Approximately 2.5%-9.1% of AML patients suffer from granulocytic sarcoma (GS), also termed myeloid sarcoma, which is a solid tumor composed of mature or immature myeloid cells[2]. The most frequent sites of GS are the bones. GS of the brain and spinal cord is rare. An intraspinal space-occupying lesion caused by GS is even rarer. The treatment regimen for AML is induction chemotherapy to obtain complete remission, followed by consolidation and reinforcement treatment or stem cell transplantation[3]. We here describe a patient with GS-associated spinal cord compression who was later diagnosed with AML during preoperative multidisciplinary consultation by hematology, oncology, imaging, and neurosurgery experts prior to emergency surgery.
A 15-year-old male patient was admitted to hospital due to 10 d of lower back pain and 4 d of numbness and fatigue of both lower limbs.
Ten days previously, the patient suffered from persistent chest and back pain without obvious inducement and no relief after rest. The degree of pain gradually worsened and was not relieved by oral analgesics. Six days later, the patient experienced numbness and fatigue in both lower limbs that affected normal walking.
The patient had no relevant medical history.
The patient had no significant family history.
The patient had abnormal sensation below the xiphoid process. Muscle strength in both lower limbs was weak, muscular tension in both lower limbs was increased, knee jerk reflex of both lower limbs was (+++), bilateral Achilles tendon reflex was (++++), bilateral Babinski sign was positive, and bilateral ankle clonus was positive.
Preoperative routine blood tests showed abnormalities in white blood cells (23.43 × 109/L), red blood cells (2.87 × 1012/L), and platelets (101.00 × 109/L). Tests for serum tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 72-4, were all negative. The ferritin level was significantly increased (> 2000 ng/mL) (Table 1).
| Inspection name | Result | Unit | Reference range |
| White blood cell | 23.43 | 109/L | 3.50-9.50 |
| Red blood cell | 2.87 | 1012/L | 4.30-5.80 |
| Hematocrit | 30.30 | % | 40.00-50.00 |
| Platelet | 101 | 109/L | 125-350 |
| CEA | 0.61 | ng/mL | 0-8.71 |
| AFP | 2.19 | ng/mL | 0-13.22 |
| Fetoprotein | > 2000 | ng/mL | 30-400 |
| CA 19-9 | < 0.6 | U/mL | 0-37.0 |
| CA 72-4 | 1.090 | U/mL | 0-6.900 |
| Procalcitonin | 0.164 | ng/mL | 0-0.500 |
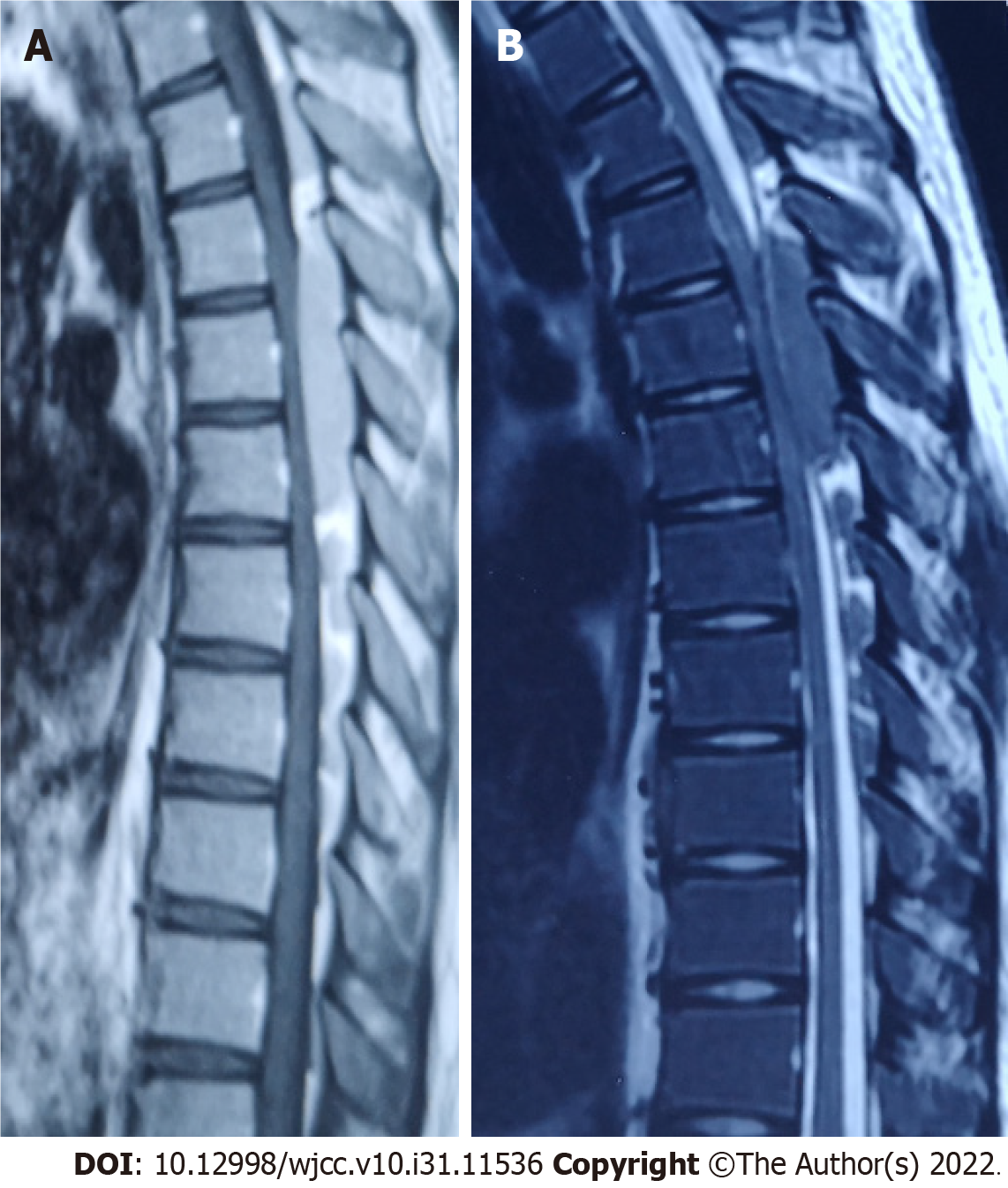
Magnetic resonance imaging showed a T3-8 spinal space-occupying lesion and marked spinal cord compression (Figure 1).
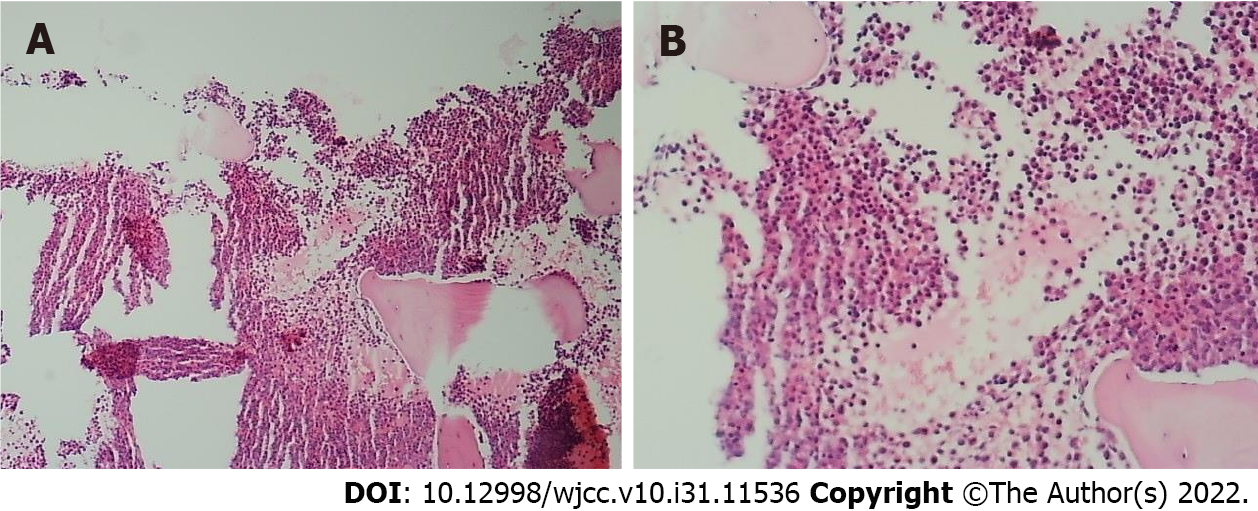
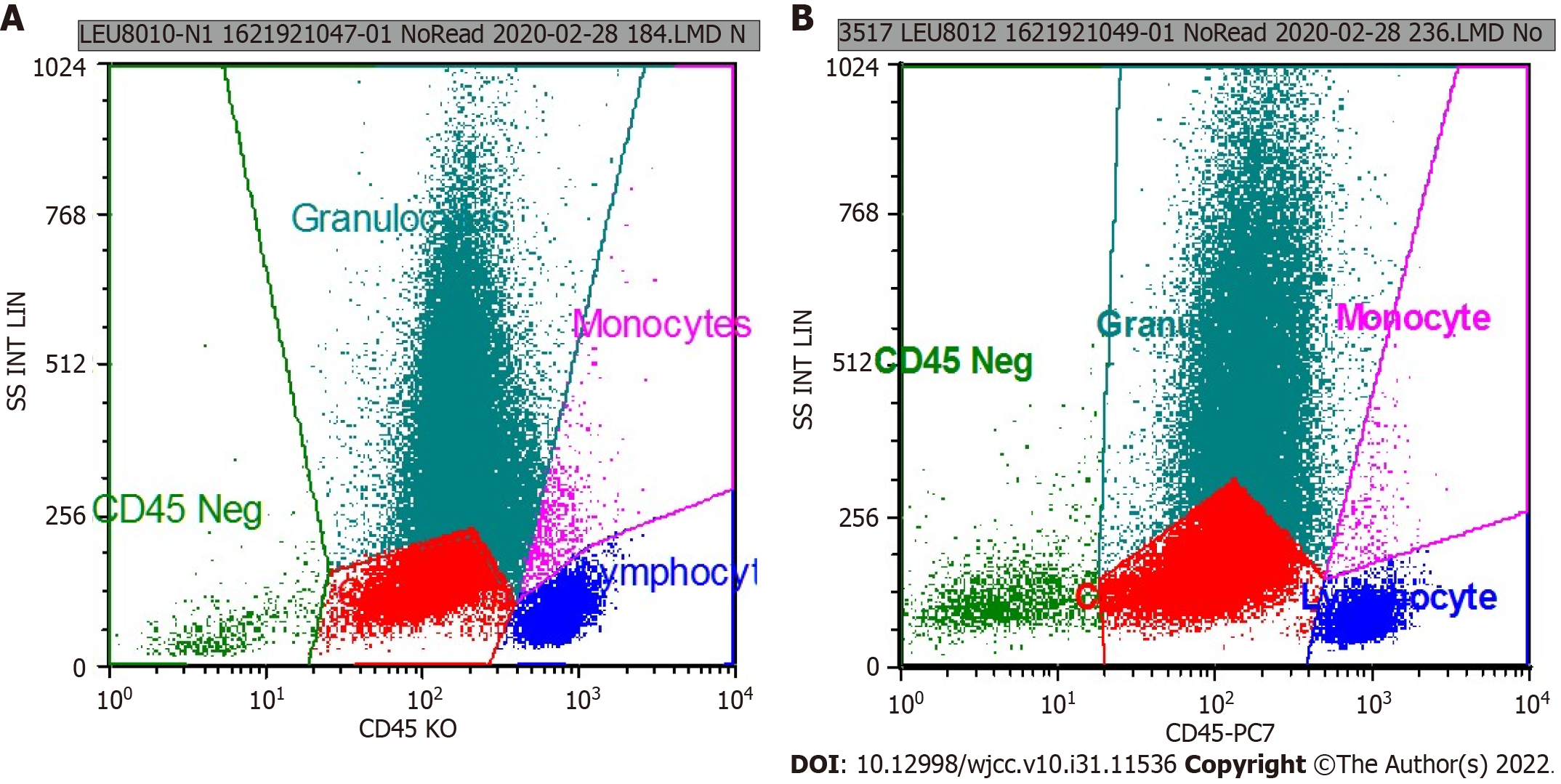
Doctors from the Department of Hematology suggested the possibility of hematological diseases and extramedullary hematopoiesis and advised bone puncture and systemic treatment. Considering the long space-occupying segment, intraoperative bleeding, and short medical history, the patient’s family refused surgical treatment and agreed to transfer him to the Department of Hematology for bone puncture. The immunohistochemical results were as follows: CD34 small vessels (+), a few round nucleus cells (+), CD117 (+), CD61 megakaryocytes (+), monocytes (+) myeloperoxidase (+), and Lyso (+); CD3 was positive occasionally; and CD20 was positive occasionally. The results of flow cytometry showed that CD117+ cells accounted for 24.61% of the total number of nuclear cells, and the immunophenotypes were CD13+, CD33+, CD34+, CD38+, CD56+, CD117+, HLA-DR+, myeloperoxidase +, CD15-, CD64-, CD7-, CD11b-, CD19-, CD10-, and CD16-. The relative proportion of granulocytes was normal. The immunophenotypes of CD13, CD16, CD15, and CD11b were disordered and the expression of CD38 and CD56 was abnormal (Figures 2 and 3).
According to the above test results combined with clinical manifestations, the patient was diagnosed with AML-M2b.
Systemic chemotherapy (IA scheme: Idarubicin + cytosine arabinoside) was administered. During treatment, the patient’s neurological function in the lower limbs was closely observed and gradually improved.
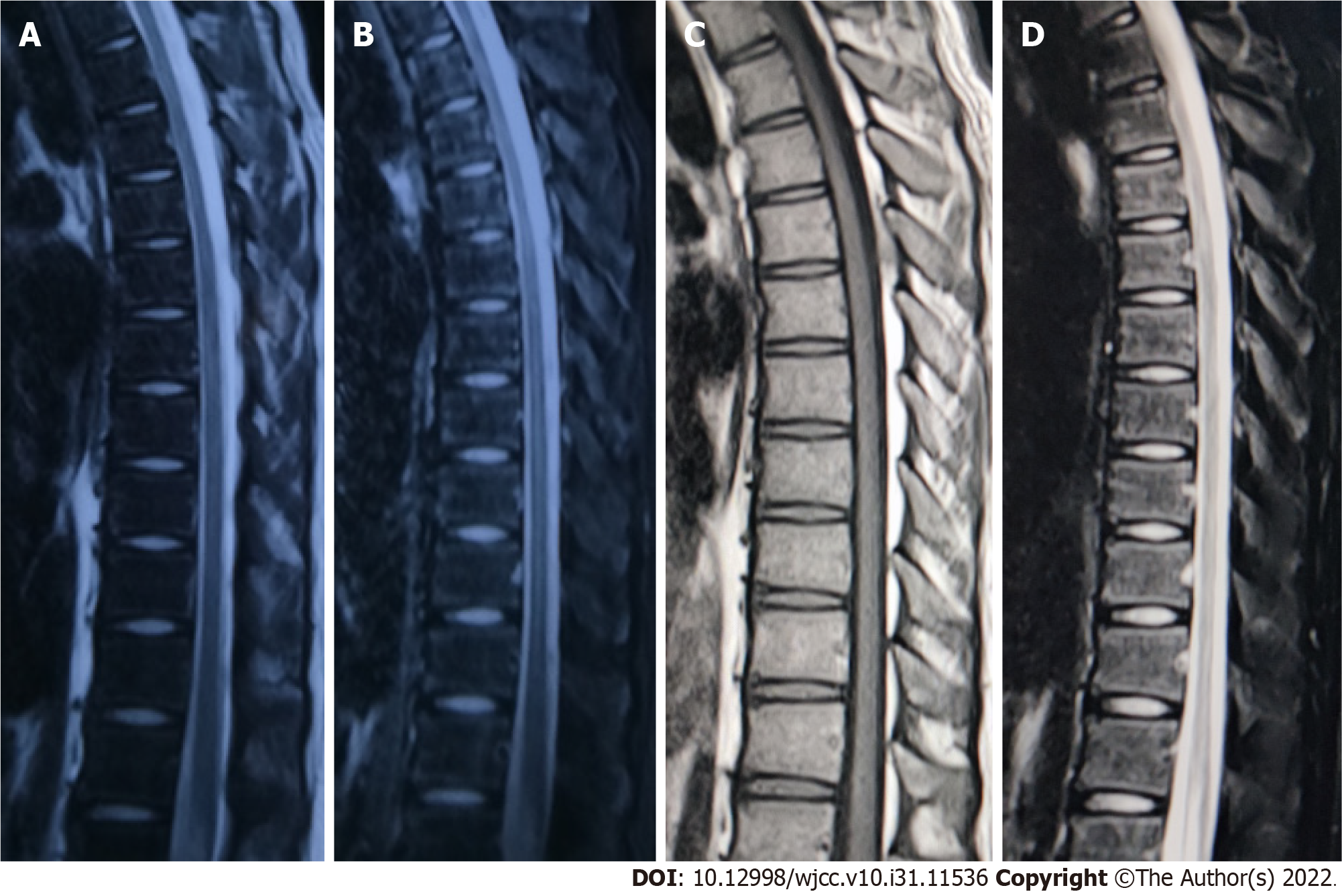
Lower limb muscle strength gradually returned to normal, chest and back pain disappeared, and the patient’s body temperature was normal. One month after treatment and 1 year after treatment (Figure 4), magnetic resonance imaging showed that the intraspinal space-occupying lesions had completely disappeared.
As a form of myeloid leukemia, GS is a localized infiltration of abnormal white blood cells in subperiosteal or soft tissue[4]. It is a localized solid tumor formed by immature granulocytes outside the bone marrow, which is very rare in clinical practice[5]. The central nervous system, orbit, and bone marrow are common sites of GS[2]. Preauricular and submandibular lymph nodes are often enlarged. There is also green pigmentation on the surface of the skin. In the late stage of the disease, all the essential organs and limbs are involved, and patients often die of anemia or infection[6].
The incidence of GS in AML is 2.5%-9.1%[7] and is more common in male patients. The incidence of GS in chronic myeloid leukemia is lower compared with AML. In particular, GS in the central nervous system is rare, and spinal cord compression caused by GS is even rarer[4]. Cord compression mainly occurs in the thoracic spinal canal. In previous literature reports[8], most patients developed intraspinal compression symptoms after the diagnosis of AML or chronic myeloid leukemia and were diagnosed as intraspinal space-occupying lesions by magnetic resonance imaging examination[6].
Most of these cases were treated with intraspinal decompression surgery, and the symptoms of intraspinal compression were significantly relieved[4]. AML patients often have solid tumors in other areas, especially intraspinal tumors, and these tumors often develop rapidly during the treatment of leukemia or when the disease is stable[9,10]. In order to relieve pain and preserve spinal cord and nerve function, spinal surgeons often choose spinal canal decompression and tumor resection in an emergency situation. However, patients could suffer from significant blood loss during surgery due to the rich blood supply and poor blood coagulation index.
Our case further confirmed that intraspinal GS can suddenly appear in previously healthy patients who had not previously been diagnosed with hematological diseases. Our patient had a sudden onset of clinical symptoms of spinal cord compression. GS should be considered in patients with spinal canal space-occupying lesions and abnormal blood tests. Standard chemotherapy in combination with simultaneous monitoring of spinal cord function could be an alternative treatment regimen to surgery. The advantages and disadvantages of surgery in these patients require further study.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Mohey NM, Egypt; Reddy R, India S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Pelcovits A, Niroula R. Acute Myeloid Leukemia: A Review. R I Med J (2013). 2020;103:38-40. [PubMed] [Cited in This Article: ] |
| 2. | Seok JH, Park J, Kim SK, Choi JE, Kim CC. Granulocytic sarcoma of the spine: MRI and clinical review. AJR Am J Roentgenol. 2010;194:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Wong WY, Williams D, Slovak ML, Charak B, Mazumder A, Snyder D, Powars DR, Brynes RK. Terminal acute myelogenous leukemia in a patient with congenital agranulocytosis. Am J Hematol. 1993;43:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Han S, Li Y, Niu T, Wang X, Li Z, Ren X, Gao J. Granulocytic sarcoma causing long spinal cord compression: Case report and literature review. J Spinal Cord Med. 2022;45:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Zhang GN, Song SQ, Zhu Y, Shi Y, Li JM. Cervical granulocytic sarcoma. Chin Med J (Engl). 2013;126:3592. [PubMed] [Cited in This Article: ] |
| 6. | Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37:649-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Benekli M, Savaş MC, Haznedaroğlu IC, Dündar SV. Granulocytic sarcoma in acute promyelocytic leukemia. Leuk Lymphoma. 1996;22:183-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Koh HJ, Baek J, Lee MS, Park HJ. Epidural chloroma and spinal cord compression. Chin Med J (Engl). 2019;132:853-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Borthakur G, Kantarjian H. Core binding factor acute myelogenous leukemia-2021 treatment algorithm. Blood Cancer J. 2021;11:114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Shallis RM, Stahl M, Bewersdorf JP, Hendrickson JE, Zeidan AM. Leukocytapheresis for patients with acute myeloid leukemia presenting with hyperleukocytosis and leukostasis: a contemporary appraisal of outcomes and benefits. Expert Rev Hematol. 2020;13:489-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |