Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11371
Peer-review started: August 1, 2022
First decision: August 22, 2022
Revised: August 30, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: November 6, 2022
Correlation between Triglyceride (TG) and estimated glomerular filtration rate (eGFR) remains largely unknown in overweight and non-overweight patients.
To investigated the dynamic changes of eGFR and lipid profiles during 3-year tenofovir disoproxil fumarate (TDF) treatment in patients with chronic hepatitis B (CHB) and overweight.
A total of 202 CHB patients who received TDF treatment at the Third People's Hospital of Changzhou (Changzhou, China) and Nanjing Drum Tower Hospital (Nanjing, China) between January 2016 and May 2018 were retrospectively enrolled. According to the body mass index (BMI) at the initiation of TDF treatment, CHB patients were divided into overweight (BMI ≥ 25 kg/m2) and non-overweight (BMI < 25 kg/m2) groups. Logistic regression was applied for the analysis of risk factors for eGFR < 90 mL/(min·1.73 m2).
There is no significant difference in hepatitis B virus DNA (HBV DNA) negativity and hepatitis Be antigen (HBeAg) loss between patients with overweight and non-overweight (both P > 0.05). More patients in non-overweight group achieved alanine aminotransferase normalization compared with those in overweight group (χ2 = 11.036, P < 0.01). In non-overweight patients, the eGFR significantly declined in the 1st year (P < 0.01), then remained at a relatively lower level. TG significantly declined in the 2nd year (P = 0.02) and increased in the 3rd year. Moreover, TG was negatively correlated with GFR at the four-time points (P = 0.002, 0.030, 0.007, 0.008, respectively). In overweight patients, eGFR and TG remained relatively stable during the 3-year treatment, and eGFR showed no significant relationship with TG. Moreover, multivariate analysis showed that age [P < 0.01, 95%CI (0.97-1.005)] and baseline eGFR [P < 0.01, 95%CI (5.056-33.668)] were independent risk factors for eGFR < 90 mL/(min·1.73 m2) at the 3rd year.
Dynamic changes in renal function were conversely related to TG during TDF treatment in patients with CHB and normal BMI, but not with overweight.
Core Tip: Correlation between Triglyceride (TG) and estimated glomerular filtration rate (eGFR) remains largely unknown. Our study indicated that more patients in non-overweight group achieved alanine aminotransferase normalization compared with those in overweight group (χ2 = 11.036, P < 0.01). In non-overweight patients, TG was negatively correlated with GFR at the four-time points (P = 0.002, 0.030, 0.007, 0.008, respectively). Dynamic changes in renal function were conversely related to TG during TDF treatment in patients with CHB and normal BMI, but not with overweight. Age [P < 0.01, 95%CI (0.97–1.005)] and baseline eGFR [P < 0.01, 95%CI (5.056–33.668)] were independent risk factors for eGFR < 90 mL/(min·1.73 m2) at the 3rd year.
- Citation: Liu SQ, Zhang XJ, Xue Y, Huang R, Wang J, Wu C, He YS, Pan YR, Liu LG. Dynamic changes of estimated glomerular filtration rate are conversely related to triglyceride in non-overweight patients . World J Clin Cases 2022; 10(31): 11371-11380
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11371.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11371
Approximately 296 million people worldwide are chronically infected with hepatitis B virus (HBV), while an estimated 820000 patients die on a yearly basis[1]. In addition, liver cirrhosis (LC) and hepatocellular carcinoma (HCC) have been identified as the main causes of death. Previous studies have shown that antiviral therapy can slow the progression of LC and reduce the incidence of HCC caused by HBV infection[2].
Tenofovir disoproxil fumarate (TDF) is recommended as one of the first-line antiviral agents. In phase III clinical trial, TDF was showed to be well tolerated during more than 7 years of follow-up[3]. In addition, the estimated glomerular filtration rate (eGFR) remained stable in patients with impaired kidney function during 3-year real-world studies conducted in Europe[4]. Our previous study also showed that eGFR remained stable irrespective of prior LC or age > 65 years old in a two-year real-world study[5]. However, potential nephrotoxicity during long-term treatment of TDF remains a concern in clinical practice. A real-world observational study reported that eGFR significantly declined during 48-mo TDF treatment, and the cumulative incidence of renal function impairment was significantly higher in the TDF group[6]. TDF therapy and underlying chronic kidney diseases were identified as independent risk factors for renal dysfunction[6]. Another study with a 10-year follow-up showed that the cumulative incidence of renal impairment was higher during TDF treatment as compared with entecavir[7].
Overweight is a global pandemic associated with dyslipidemia. The accumulation of triglycerides leads to the development of hepatic steatosis (HS), resulting in a high incidence of non-alcoholic fatty liver[8]. Individuals may concomitantly suffer from two liver diseases, which in turn may have a synergistic effect on the risk of HCC, cirrhosis, and death[9]. Compared with non-overweight patients, those suffering from overweight and dyslipidemia had significantly lower average eGFR[10].
In addition, lipid profiles were regulated by TDF in patients with human immunodeficiency virus (HIV) or hepatitis B virus (HBV) infection. Triglyceride (TG), total cholesterol, and high-density lipoprotein cholesterol (HDL-C) significantly increased after switching from TDF to TAF, thus significantly worsening the lipid profile[11-14]. For patients with CHB, TDF but not entecavir significantly decreases serum lipoprotein, including HDL-C, low-density lipoprotein cholesterol (LDL-C), and total cholesterol (CHOL)[15]. However, the effects of TDF on eGFR and lipid profiles in patients CHB and obese remain largely unknown.
Herein, we investigated the dynamic changes of eGFR and lipid profiles during 3-year TDF treatment and their correlations in patients with CHB and overweight.
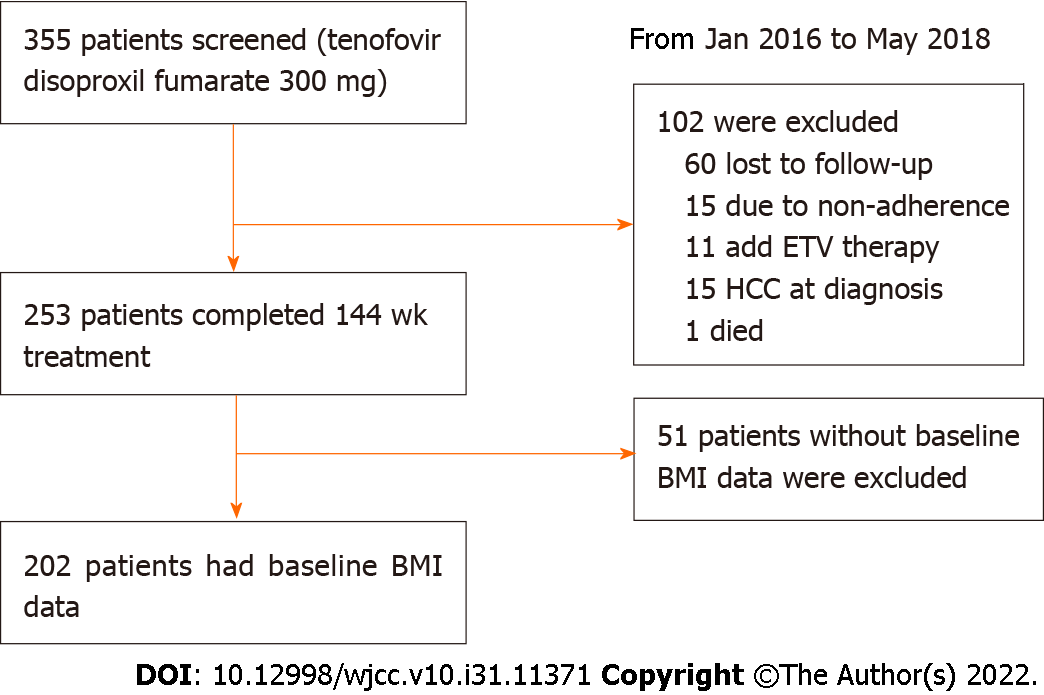
A total of 355 HBV-infected patients who received TDF treatment at the Third People's Hospital of Changzhou (Changzhou, China) and Nanjing Drum Tower Hospital (Nanjing, China) between January 2016 and May 2018 were retrospectively enrolled. CHB and LC were diagnosed according to the Chinese guidelines for the prevention and treatment of CHB (2019 version)[16]. Patients with immunodeficiency diseases, autoimmune diseases, alcohol abuse, and co-infection with other hepatitis viruses, were excluded. After 3-year follow-up, 153 patients with poor compliance, TDF discontinuation, or missing data were additionally excluded (Figure 1).
Demographic and clinical data including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), CHOL, TG, creatinine (Cr), calcium (Ca), phosphorus (P), and HBeAg were collected., complete response rates for HBV DNA suppression and ALT normalization were calculated. According to the International Obesity Task Force, Asians with body mass index (BMI) ≥ 25 kg/m² were diagnosed with overweight[17].
The study was approved by the Ethics Committee of the Third People’s Hospital of Changzhou according to the Declaration of Helsinki 1975. The retrospective study was non-interventional, anonymous and harmless to the patients, the written informed consents were exempt according to the ethic approval.
eGFR was calculated based on the chronic kidney disease Epidemiology Collaboration equation (CKD-EPIcr). Decreased eGFR was defined as eGFR < 90 mL/(min·1.73 m2).
All data were analyzed using SPSS26.0 (Chicago, IL, United States). For continuous variables, data were expressed as median (interquartile range, IQR) and compared using the Mann-Whitney U test. Categorical variables are expressed as frequencies and compared using the Chi-square test. Spearman correlation was used to analyze the correlation between eGFR and lipid profiles. Multivariate logistic regression was used to analyze the risk factors for decreased eGFR at the end of a 3-year follow-up. A two-sided P < 0.05 indicated statistical significance.
Among the 202 CHB patients, 44 patients (21.8%) were overweight (BMI ≥ 25 kg/m2). As shown in Table 1, there were more male patients in the overweight group (88.6% vs 70.3%, P = 0.01). TG and CHOL levels were comparable between two groups (P = 0.13 and 0.98, respectively). There was no significant difference in ALT, AST, TBIL, eGFR between the two groups (all P > 0.05).
| Variables | BMI < 25 (n = 158) | BMI ≥ 25 (n = 44) | Z or χ2 | P value |
| Age (yr) | 36.0 (32.0-47.3) | 38.0 (32.3-45.8) | -0.098 | 0.92 |
| Male, n (%) | 111 (70.3) | 39 (88.6) | 6.084 | 0.01 |
| ALT (U/L) | 59.7 (29.3-163.5) | 52.6 (36.0-189.6) | -0.684 | 0.49 |
| AST (U/L) | 40.6 (28.2-92.5) | 44.0 (25.4-104.3) | -0.222 | 0.83 |
| TBIL (µmol/L) | 14.1 (10.7-20.8) | 16.0 (12.3-18.9) | -1.328 | 0.18 |
| CHOL (mmol/L) | 3.9 (3.2-4.4) | 3.8 (3.2-4.4) | -0.023 | 0.98 |
| TG (mmol/L) | 0.9 (0.6-1.2) | 1.0 (0.7-1.4) | -1.508 | 0.13 |
| Cr (µmol/L) | 69.0 (59.8-81.0) | 77.0 (62.0-85.2) | -2.342 | 0.02 |
| eGFR (mL/(min·1.73 m2) | 112.5 (97.0-131.4) | 105.9 (96.4.3-133.9) | -0.951 | 0.18 |
| eGFR categories, n (%) | ||||
| < 90 | 22 (13.9) | 10 (22.7) | ||
| ≥ 90 | 136 (86.0) | 34 (77.3) | 2.001 | 0.16 |
| LC, n (%) | 27 (17.1) | 7 (20.6) | 0.034 | 0.85 |
| Diabetes mellitus, n (%) | 4 (2.5) | 2 (4.5) | 0.484 | 0.49 |
| Kidney disease, n (%) | 1 (0.6) | 0 (0) | 0.28 | 0.6 |
| HBV DNA (Log10IU/mL) | 5.06 (3.72-6.86) | 5.17 (3.2-6.97) | -0.091 | 0.93 |
| HBeAg, n (%) | 112 (70.9) | 33 (75.0) | 0.288 | 0.59 |
In non-overweight group, the median HBV viral load at baseline was 5.06 Log10IU/mL, 152 (96.2%) patients had undectable HBV DNA at week 144, and 138 (87.3%) patients achieved ALT normalization. For patients with overweight, 41 (93.2%) patients had undectable HBV DNA at week 144, and 29 (65.9%) patients achieved ALT normalization. At week 144, among the 112 patients with positive HBeAg in non-overweight group, 50 (44.6%) and 6 (5.4%) experienced HBeAg loss and seroconversion. In overweight patients, 33 (75%) were HBeAg-positive at baseline, 14 (42.4%) and 2 (6.1%) experienced HBeAg loss and seroconversion. No patient had HBsAg loss in either non-overweight or overweight patients during 144-wk follow-up. There is no significant difference in HBV DNA negativity and HBeAg loss between patients with overweight and non-overweight (both P > 0.05). More patients in non-overweight group achieved ALT normalization compared with those in overweight group (χ2 = 11.036, P < 0.01).
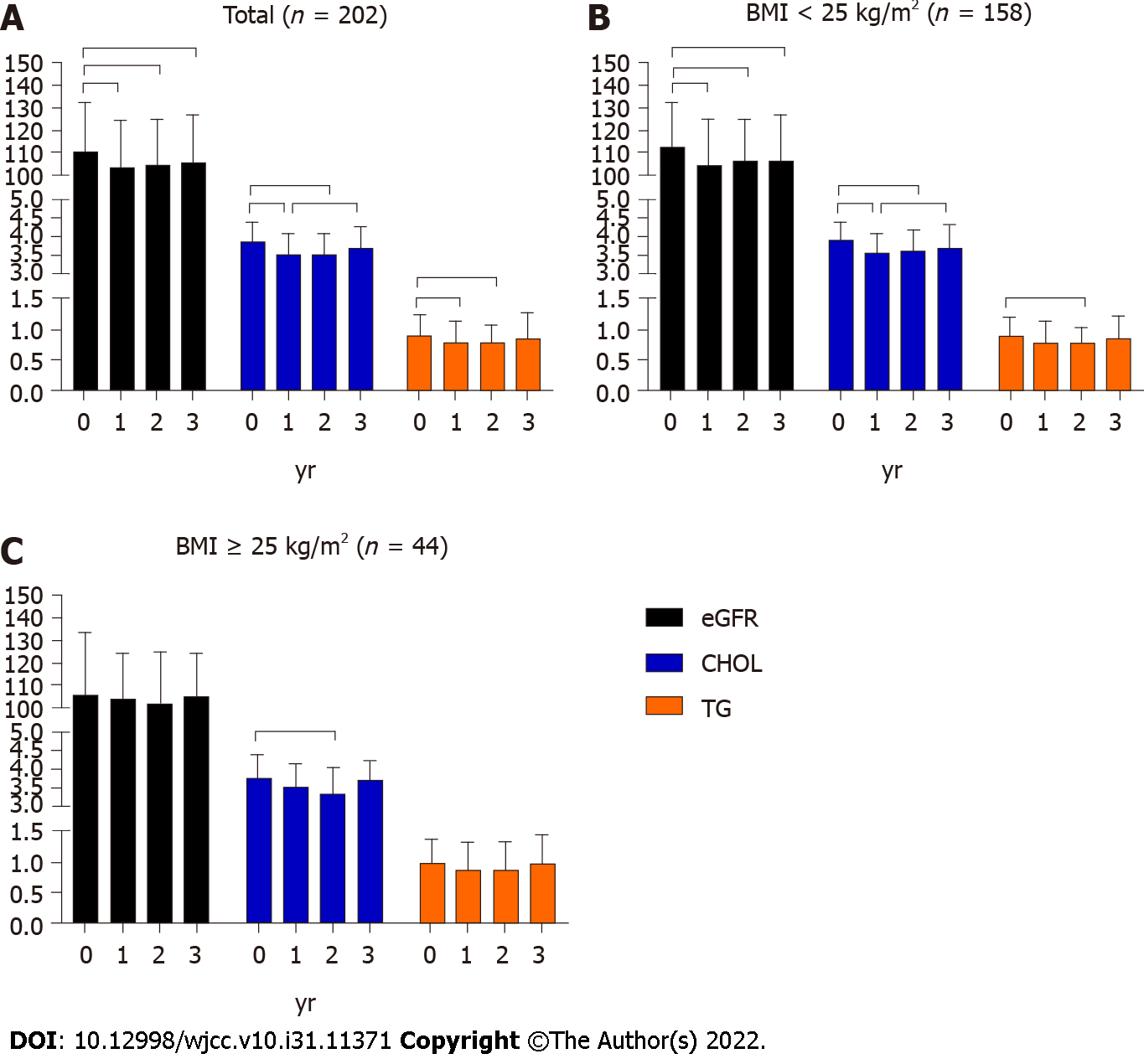
As shown in Figure 2, in non-overweight patients, eGFR significantly declined in the 1st year and remained as such until the 3rd year. Among the 136 patients who had eGFR ≥ 90 mL/(min·1.73 m2) at baseline, 20 (14.7%) had decreased eGFR at the 3rd year. While in overweight patients, eGFR remained relatively stable during the 3-year treatment. Among the 34 patients who had eGFR ≥ 90 mL/(min·1.73 m2) at baseline, two (5.9%) patients had decreased eGFR at the 3rd year, and the difference was not significant from patients in non-obese group (P = 0.25).
In non-overweight patients, CHOL significantly decreased in the 1st year (P < 0.05), after which it slowly increased. Unexpectedly, CHOL returned to the baseline level in the 3rd year. In overweight patients, CHOL significantly decreased in the 2nd year (P < 0.05) and then returned to the baseline level at the 3rd year.
In non-overweight patients, TG significantly declined in the 2nd year (P < 0.05) and increased at the 3rd year, while in overweight patients, TG remained relatively stable during the 3-year follow-up.
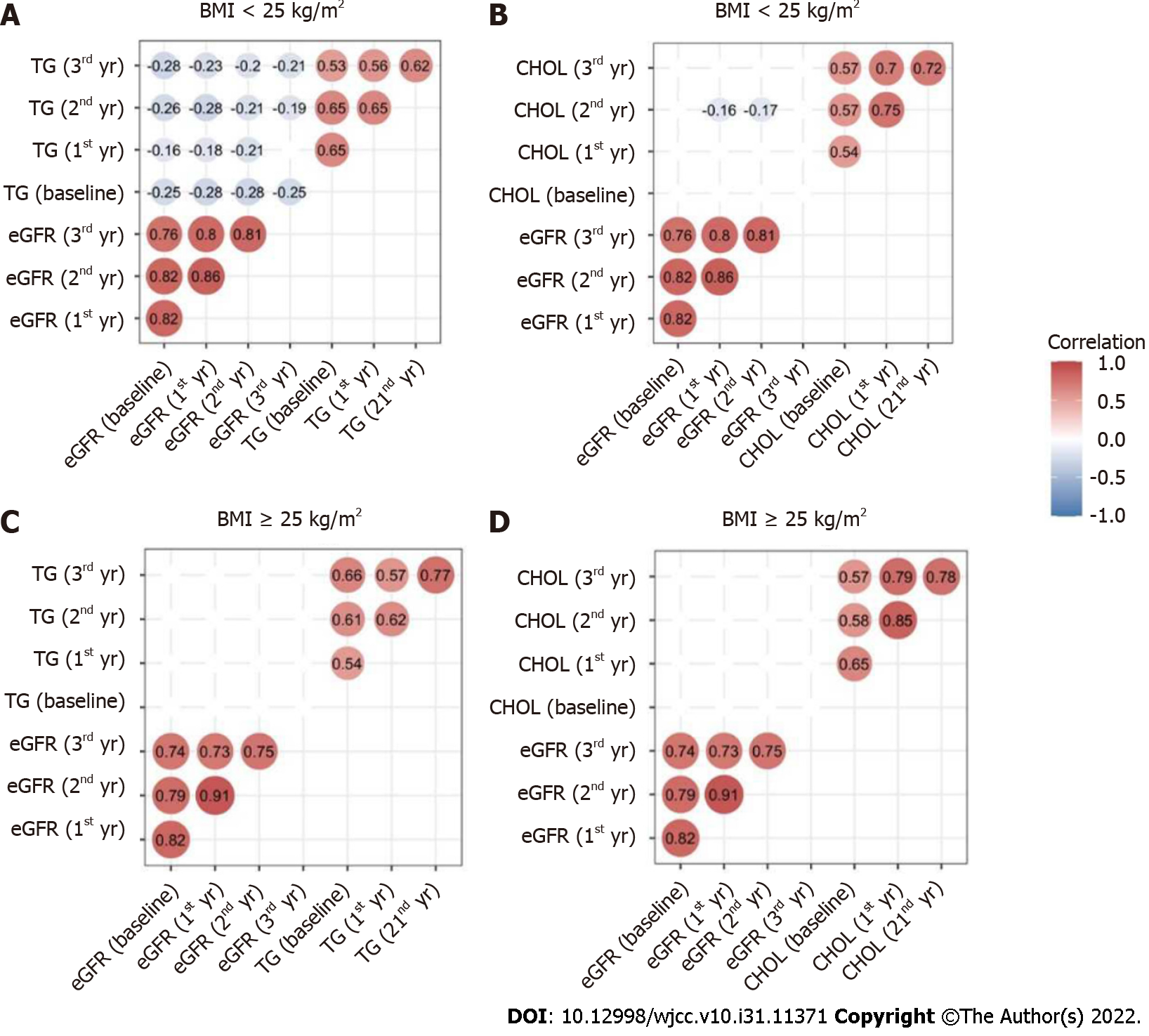
In all the patients, baseline eGFR was positively correlated with eGFR at the 1st, 2nd, and 3rd year. There were also positive relationships between TG at the four-time points. Similar relationships were found in CHOL.
In non-overweight patients, eGFR was negatively correlated with TG at each time point (all P < 0.05) (Figure 3A). In addition, eGFR did not correlate with CHOL at each time-point, except for the 2nd year, while in overweight patients, eGFR showed no significant relationship with TG or CHOL at any time-point.
Univariate and multivariate logistic regression analyses were performed to analyze the risk factors for decreased eGFR at the end of the 3rd year. For the 202 patients, age, LC, TBil, and baseline eGFR < 90 mL/(min·1.73 m2) were associated with decreased eGFR in the 3rd year. Next, multivariate analysis showed that age (P < 0.01) and baseline eGFR < 90 mL/(min·1.73 m2) (P < 0.01) were independent risk factors for eGFR < 90 mL/(min·1.73 m2) in the 3rd year (Table 2).
| Baseline variables | Univariate analysis | Multivariate analysis | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age | 0.909 | 0.878-0.941 | < 0.01 | 0.93 | 0.890-0.971 | 0.01 |
| Gender (male) | 0.576 | 0.248-1.335 | 0.2 | |||
| BMI value | 0.949 | 0.884-1.067 | 0.38 | |||
| LC | 6.144 | 2.781-13.574 | < 0.01 | 2.07 | 0.684-6.265 | 0.2 |
| ALT | 1.001 | 0.999-1.002 | 0.57 | |||
| AST | 1 | 0.998-1.003 | 0.77 | |||
| TBIL | 0.975 | 0.956-0.994 | 0.01 | 0.989 | 0.972-1.007 | 0.24 |
| CHOL | 1.149 | 0.762-1.732 | 0.51 | |||
| TG | 0.795 | 0.475-1.332 | 0.38 | |||
| eGFR < 90 | 14.8 | 6.192-35.376 | < 0.01 | 13.304 | 5.084-34.812 | < 0.01 |
For 158 non-overweight patients, univariate analysis showed that age, LC, TBil, and baseline eGFR < 90 mL/(min·1.73 m2) were associated with decreased eGFR in the 3rd year. Moreover, multivariate analysis showed that age (P < 0.01) and baseline eGFR < 90 mL/(min·1.73 m2) (P < 0.01) were independent risk factors for decreased eGFR in the 3rd year (Table 3).
| Baseline variables | Univariate analysis | Multivariate analysis | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age | 0.912 | 0.879-0.946 | < 0.01 | 0.934 | 0.891-0.979 | < 0.01 |
| Gender (male) | 0.519 | 0.209-1.289 | 0.16 | |||
| LC | 6.937 | 2.832-16.996 | < 0.001 | 1.823 | 0.532-6.254 | 0.34 |
| ALT | 1.001 | 0.998-1.004 | 0.46 | |||
| AST | 1.001 | 0.950-0.992 | 0.59 | |||
| TBIL | 0.971 | 0.951-0.992 | < 0.01 | 0.989 | 0.971-1.008 | 0.25 |
| CHOL | 1.021 | 0.652-1.601 | 0.93 | |||
| TG | 0.599 | 0.310-1.158 | 0.13 | |||
| eGFR < 90 | 12.429 | 4.504-34.392 | < 0.01 | 9.902 | 3.273-29.955 | < 0.01 |
For 44 overweight patients, age, LC, and baseline eGFR < 90 mL/(min·1.73 m2) (all P < 0.01) were associated with decreased eGFR in the 3rd year, and baseline eGFR < 90 mL/(min·1.73 m2) was the independent risk factor for decreased eGFR in the 3rd year (Table 4).
| Baseline variables | Univariate analysis | Multivariate analysis | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age | 0.892 | 0.811-0.980 | 0.02 | 0.884 | 0.773-1.010 | 0.07 |
| Gender (male) | 0.965 | 0.095-1.412 | 0.27 | |||
| LC | 3.875 | 0.685-21.934 | 0.13 | |||
| ALT | 1 | 0.997-1.005 | 0.95 | |||
| AST | 0.999 | 0.942-1.003 | 0.71 | |||
| TBIL | 0.997 | 0.942-1.005 | 0.92 | |||
| CHOL | 1.967 | 0.710-5.452 | 0.19 | |||
| TG | 1.337 | 0.402-4.717 | 0.61 | |||
| eGFR < 90 | 0.027 | 0.004-0.192 | < 0.01 | 0.025 | 0.003-0.235 | 0.01 |
Data from the present study indicated that it was not sufficient to withdraw TDF according to fluctuations in eGFR. As the dynamic changes of eGFR and lipid profile were different in patients with overweight or non-overweight, BMI and lipid profiles should also be taken into consideration. eGFR remained stable during the 3-year treatment, which is especially true for overweight patients. However, BMI was not an independent risk factor for decreased eGFR at the end of 3-year TDF treatment, BMI should be monitored during treatment. As there is evidence that TDF may be associated with weight loss in patients with HIV infection[18], it would be interesting to monitor the weight in patients with HBV infection and TDF treatment. However, BMI was not calculated during the follow-up in the present study.
While there is increasing evidence supporting the regulation of lipid profiles by TDF[19-21], the mechanisms remain largely unknown. Recently, Suzuki et al[22] reported that TDF, but not ETV, reduced supernatant total CHOL, LDL-C, HDL-C, and TG by up-regulating hepatic CD36 in HepG2 cells. Other mechanisms remain to be elucidated, regardless of food intake. It has been reported that increased LDL-C is associated with eGFR decline and the development of chronic kidney deficiency in men without hypertension or diabetes[23]. To the best of our knowledge, there are still barriers to the implementation of LDL-C in many rural areas. Moafi et al[24] reported that CHOL, HDLC, and TG were negatively correlated with eGFR after adjusting BMI, blood pressure, and blood glucose. In the present study, it was interesting to find that TG was negatively correlated with eGFR in non-overweight. Thus, TG regulation may be beneficial for reducing the renal toxicity of TDF.
A Korean study showed that advanced age was associated with reduced renal function at week 144. In addition, comorbidities including diabetes or hypertension showed the tendency toward renal impairment[25]. Similar results were found in the present study, where age and baseline eGFR < 90 mL/(min·1.73 m2) were independent risk factors for reduced renal function. In clinical practice, it is worrisome to administer TDF in patients with impaired eGFR. Unexpectedly, patients with impaired baseline eGFR had relatively stable eGFR during 3-year TDF treatment. This result suggests that TDF remains a useful alternative in patients with decreased eGFR, although routine tests are recommended.
This study has several limitations. First, lipid indicators, including LDL and HDL, are lacking. Second, other indicators, including urine protein quantitation Cystatin C, which may be more sensitive, were not detected during TDF treatment.
In conclusion, dynamic changes in renal function were associated with TG during TDF treatment in CHB patients without overweight, but not with overweight.
Tenofovir disoproxil fumarate (TDF) is recommended as one of the first-line antiviral agents. The effects of TDF on lipid profiles in patients with chronic hepatitis B (CHB) and overweight are largely unknown.
Overweight is a global pandemic associated with dyslipidemia. Correlation between triglyceride (TG) and estimated glomerular filtration rate (eGFR) remains largely unclear.
To determine the impact of 3-year TDF treatment on lipid metabolism profiles and renal function in Chinese patients with CHB and overweight.
This multi-centre, retrospective cohort study included CHB patients who received TDF treatment. According to the body mass index (BMI) at the initiation of TDF treatment, CHB patients were divided into different groups. Changes of lipid profiles and renal function, as well as the risk factors for eGFR < 90 mL/(min·1.73 m2) were analyzed. Spearman correlation was used to analyze the correlation between eGFR and lipid profiles.
In non-overweight patients, TG was negatively correlated with GFR at the four-time points (P = 0.002, 0.030, 0.007, 0.008, respectively).
There is a negative relation between TG and changes in eGFR during TDF treatment in patients with CHB and normal BMI.
TG regulation may be beneficial for reducing the renal toxicity of TDF.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, United States; Jandale OA, Syria; Sahin TT, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | World Health Organization. Fact sheet of Hepatitis B 2021 [cited May 12, 2022]. Available from: www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Cited in This Article: ] |
| 2. | Pisano MB, Giadans CG, Flichman DM, Ré VE, Preciado MV, Valva P. Viral hepatitis update: Progress and perspectives. World J Gastroenterol. 2021;27:4018-4044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (9)] |
| 3. | Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P, Kitrinos KM, Subramanian GM, Gane E, Marcellin P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 4. | Marcellin P, Zoulim F, Hézode C, Causse X, Roche B, Truchi R, Pauwels A, Ouzan D, Dumortier J, Pageaux GP, Bourlière M, Riachi G, Zarski JP, Cadranel JF, Tilliet V, Stern C, Pétour P, Libert O, Consoli SM, Larrey D. Effectiveness and Safety of Tenofovir Disoproxil Fumarate in Chronic Hepatitis B: A 3-Year, Prospective, Real-World Study in France. Dig Dis Sci. 2016;61:3072-3083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Zheng S, Liu L, Lu J, Zhang X, Shen H, Zhang H, Xue Y, Lin L. Efficacy and safety of tenofovir disoproxil fumarate in Chinese patients with chronic hepatitis B virus infection: A 2-year prospective study. Medicine (Baltimore). 2019;98:e17590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47:1673-1681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Mak LY, Hoang J, Jun DW, Chen CH, Peng CY, Yeh ML, Kim SE, Huang DQ, Jeong JY, Yoon E, Oh H, Tsai PC, Huang CF, Ahn SB, Trinh H, Xie Q, Wong GLH, Enomoto M, Shim JJ, Lee DH, Liu L, Kozuka R, Cho YK, Jeong SW, Kim HS, Trinh L, Dao A, Huang R, Hui RW, Tsui V, Quek S, Khine HHTW, Ogawa E, Dai CY, Huang JF, Cheung R, Wu C, Chuang WL, Lim SG, Yu ML, Yuen MF, Nguyen MH. Longitudinal renal changes in chronic hepatitis B patients treated with entecavir versus TDF: a REAL-B study. Hepatol Int. 2022;16:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Wang X, Rao H, Liu F, Wei L, Li H, Wu C. Recent Advances in Adipose Tissue Dysfunction and Its Role in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, Janssen HLA, de Knegt RJ, Sonneveld MJ. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Wang X, Wang H, Li J, Gao X, Han Y, Teng W, Shan Z, Lai Y. Combined Effects of Dyslipidemia and High Adiposity on the Estimated Glomerular Filtration Rate in a Middle-Aged Chinese Population. Diabetes Metab Syndr Obes. 2021;14:4513-4522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lim J, Choi WM, Shim JH, Lee D, Kim KM, Lim YS, Lee HC, Choi J. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in treatment-naïve chronic hepatitis B. Liver Int. 2022;42:1517-1527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Mallon PWG, Brunet L, Fusco JS, Prajapati G, Beyer A, Fusco GP, Wohlfeiler MB. Lipid Changes After Switch From TDF to TAF in the OPERA Cohort: LDL Cholesterol and Triglycerides. Open Forum Infect Dis. 2022;9:ofab621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Plum PE, Maes N, Sauvage AS, Frippiat F, Meuris C, Uurlings F, Lecomte M, Léonard P, Paquot N, Fombellida K, Vaira D, Moutschen M, Darcis G. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis. 2021;21:910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Suzuki K, Suda G, Yamamoto Y, Abiko S, Kinoshita K, Miyamoto S, Sugiura R, Kimura M, Maehara O, Yamada R, Kitagataya T, Shigesawa T, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Sakamoto N. Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on lipid profiles in patients with hepatitis B. PLoS One. 2022;17:e0261760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Shaheen AA, AlMattooq M, Yazdanfar S, Burak KW, Swain MG, Congly SE, Borman MA, Lee SS, Myers RP, Coffin CS. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther. 2017;46:599-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 90] [Reference Citation Analysis (0)] |
| 17. | World Health Organization. The Asia-pacific perspective: redefining obesity and its treatment International Association for the Study of Obesity: International Obesity Taskforce; 2021 . [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Shah S, Pilkington V, Hill A. Is tenofovir disoproxil fumarate associated with weight loss? AIDS. 2021;35:S189-S195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Yazie TS. Dyslipidemia and Associated Factors in Tenofovir Disoproxil Fumarate-Based Regimen Among Human Immunodeficiency Virus-Infected Ethiopian Patients: A Hospital-Based Observational Prospective Cohort Study. Drug Healthc Patient Saf. 2020;12:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yang J, Chen J, Ji Y, Tang Q, Zhang R, Liu L, Shen Y, Xun J, Song W, Tang Y, Wang Z, Qi T, Lu H. Lipid profile and renal safety of tenofovir disoproxil fumarate-based anti-retroviral therapy in HIV-infected Chinese patients. Int J Infect Dis. 2019;83:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Tungsiripat M, Kitch D, Glesby MJ, Gupta SK, Mellors JW, Moran L, Jones L, Alston-Smith B, Rooney JF, Aberg JA. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24:1781-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Suzuki K, Suda G, Yamamoto Y, Furuya K, Baba M, Nakamura A, Miyoshi H, Kimura M, Maehara O, Yamada R, Kitagataya T, Yamamoto K, Shigesawa T, Ohara M, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ogawa K, Ohnishi S, Sakamoto N; NORTE Study Group. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J Gastroenterol. 2021;56:168-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 23. | Kuma A, Uchino B, Ochiai Y, Kawashima M, Enta K, Tamura M, Otsuji Y, Kato A. Impact of low-density lipoprotein cholesterol on decline in estimated glomerular filtration rate in apparently healthy young to middle-aged working men. Clin Exp Nephrol. 2018;22:15-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Moafi M, Assadi F, Heshmat R, Khoshhali M, Qorbani M, Motlagh ME, Dashti R, Taheri M, Kelishadi R. Impact of dyslipidemia on estimated glomerular filtration rate in apparently healthy children and adolescents: the CASPIAN-V study. World J Pediatr. 2019;15:471-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kwon JH, Song MJ, Jang JW, Bae SH, Choi JY, Yoon SK, Kim HY, Kim CW, Song DS, Chang UI, Yang JM, You CR, Choi SW, Lee HL, Lee SW, Han NI, Nam SW, Kim SG, Kim YS, Kim SH, Lee BS, Lee TH, Cho EY. Efficacy and Safety of Tenofovir Disoproxil Fumarate in Treatment-Naïve Patients with Chronic Hepatitis B in Korea. Dig Dis Sci. 2019;64:2039-2048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |