Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11226
Peer-review started: June 29, 2022
First decision: August 1, 2022
Revised: August 19, 2022
Accepted: September 27, 2022
Article in press: September 27, 2022
Published online: November 6, 2022
Obesity is becoming an inevitable pandemic all over the world. The World Obesity Federation predicts in the 2022 World Obesity Atlas that one billion people worldwide, including 1 in 5 women and 1 in 7 men, will be living with obesity by 2030. Moreover, the prevalence of diabetes is increasing worldwide, and diabetes is becoming more of a public health problem. Increased insulin resistance due to obesity and deficiency in insulin secretion are the two main causes of type 2 diabetes mellitus (T2DM). An exogenous chemical or mixture of chemicals that interferes with any aspect of hormone action was defined as endocrine-disrupting chemicals (EDCs). Bisphenol A (BPA), the first known EDC, was synthesized and was considered to be estrogenic. Global production of BPA has increased progressively from 5 to 8 million tons (MT) between 2010 and 2016. Furthermore, researchers estimated that the production should reach 10.2 MT by 2022. The human population is exposed to EDCs in daily life in such forms as pesticides/herbicides, industrial and household products, plastics, detergents, and personal care products. The term obesogen was used for chemicals that promote weight gain and obesity by increasing the number of adipocytes and fat storage in existing adipocytes, changing the energy balance, and finally regulating appetite and satiety. Besides the obesogenic effect, EDCs can cause T2DM through alteration in ß cell function and morphology and insulin resistance. In this review, we provide clinical and mechanistic evidence regarding EDCs as obesogen and diabetogen. However, those studies are not enough methodologically to indicate causality. In this respect, randomized clinical trials are needed to investigate the association between obesogen, diabetogen and the related metabolic clinical picture.
Core Tip: An exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action was defined as Endocrine-Disrupting Chemicals (EDCs). Obesogens can promote obesity by increasing the number of adipocytes and fat storage in existing adipocytes, changing the calories burned at rest, changing the energy balance, and finally regulating satiety. Besides the obesogenic effect, EDCs can cause type 2 diabetes mellitus through alteration in ß cell function and morphology and insulin resistance. In this review, we provide clinical and mechanistic evidence regarding EDCs as obesogen and diabetogen.
- Citation: Kurşunoğlu NE, Sarer Yurekli BP. Endocrine disruptor chemicals as obesogen and diabetogen: Clinical and mechanistic evidence. World J Clin Cases 2022; 10(31): 11226-11239
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11226.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11226
Obesity has become a “preventable” pandemic affecting people of all ages around the world. Globally, obesity has nearly tripled since 1975. In 2016, 39% of adults (more than 1.9 billion) aged 18 and over were overweight and 13% (more than 650 million) were obese[1]. The World Obesity Federation predicts in the 2022 World Obesity Atlas that one billion people worldwide, including 1 in 5 women and 1 in 7 men, will be living with obesity by 2030[2]. The International Classification of Disease defines obesity as a chronic, recurrent, and multifactorial disease[3]. It is also a significant risk factor for several other non-communicable diseases (NCDs), such as metabolic diseases [e.g., type 2 diabetes mellitus (T2DM) and fatty liver], cardiovascular diseases (such as hypertension, myocardial infarction, and stroke), and some malignancies (including breast, ovarian, prostate, liver, kidney, and colorectal cancers)[4]. Obesity affects the mind and body in many ways, including hunger, satiety, metabolism, hormone balance, and body weight. These changes can remain the same for many years, even if the weight is lost. In addition, obesity is a disease that recurs frequently. This means that individuals are treated without changing the obesogenic environment and other underlying causes, and then individuals are re-exposed to the same environment[2].
Diabetes is a progressive chronic disease that occurs when the pancreas cannot produce enough insulin, or the body cannot use the produced insulin effectively. The prevalence of diabetes is increasing worldwide, and diabetes is becoming more of a public health problem.
In 2021, an estimated 537 million adults worldwide were living with diabetes[5], up from 108 million in 1980[6]. The global prevalence of diabetes has more than doubled since 1980, from 4.7% to 10.5%. In the 10th edition of the Diabetes Atlas published by the International Diabetes Federation, it is estimated that 643 million adults will be living with diabetes by 2030, and 783 million adults by 2045[5]. In addition, total health expenditures related to diabetes are projected to be $966 billion, $1028 billion, and $1054 billion in those years, respectively. Another feature of these expenditures is that the prevalence of diabetes has been increasing rapidly over the past three decades, with this increase being even faster in low- and middle-income countries.
Diabetes is treatable and its consequences can be avoided or prevented by diet, physical activity, medications, and regular check-ups. However, diabetes is still a major cause of blindness, kidney failure, lower limb amputation, and many other conditions that affect the quality of life in the long term[7].
Endocrine disruptors were defined as, “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action”, in The Endocrine Society's Second Scientific Statement on endocrine-disrupting chemicals (EDCs)[8]. The history of EDCs goes back a long time. The first known EDC, Bisphenol A (BPA), was synthesized in 1891 and was considered to be estrogenic in 1936[9]. However, we must keep in mind that by definition there can be hundreds of other EDCs.
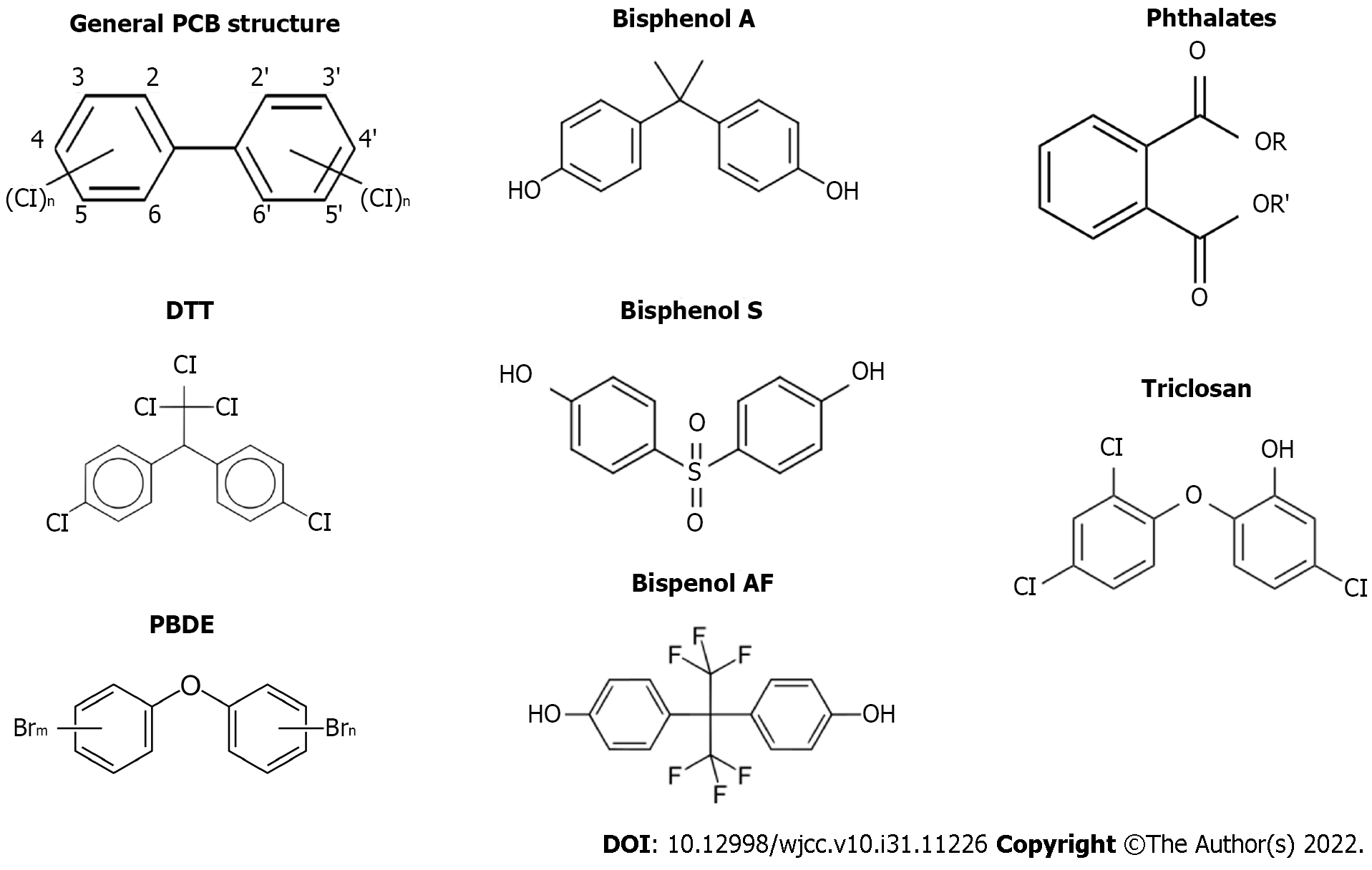
Persistent organic pollutants (POPs) are a group of chemicals that include pesticides (DDT, HCB) and industrial chemicals, for instance, polychlorinated bisphenols (PCBs) (Figure 1). POPs bioaccumulate in tissues and amplify up the food chain[10]. POPs persist in the environment for long periods because they are chemically resistant to environmental degradation. They can accumulate and pass from one species to the next through the food chain[11]. Three kinds of POPs named chlorinated, hydrophobic, and brominated accumulate predominantly in adipose-rich tissues[12].
In addition, adipose tissue plays an important role in POP’s storage and toxicokinetics. Adipose tissue stores these chemicals and acts as a buffer. On the other hand, it is a source of constant internal exposure[13]. Although the use of many POPs, especially PCBs, HCB and DDT, is prohibited in most countries, exposure still exists due to their persistence in the environment[14-16].
Heavy metals are directly related to many cancers. Besides this, two heavy metals, arsenic, and cadmium, are also classified as EDC.
Mining, metallurgy, electroplating, paints, combustion fumes, and overuse of fertilizers and pesticides are the main sources of cadmium contamination[17]. As it is more mobile and soluble compared to other metals, it is quickly absorbed by plants and accumulated in their edible parts[18,19]. Cadmium began to attract attention as an EDC with the discovery of the ability of cadmium and other heavy metals to activate the estrogen receptor[20]. Initially, it was discovered that cadmium binds with high affinity to purified ERα cells and activates them[21].
Inorganic arsenic (iAs) is found naturally in soil and in ground and surface water. This exposure can vary by location and diet. So, exposure to detrimental levels can be widespread[22]. Although multiple mechanisms, including DNA damage and oxidative stress, influence toxicity, there is also some evidence pointing to the interaction of arsenic with steroid receptors[23-25]. In a broader context, these studies provide new evidence that iAs exposure targets many receptors and have many effects that can be considered as an EDC[26,27].
Non-persistent endocrine disrupting chemicals refers to a wide variety of chemical compounds used in industrial applications whose main characteristic is a shorter half-life and lower lipid solubility. Nevertheless, with constant exposure, they are continually present in biological samples of the general population[28]. They are commonly encountered in plastics, medical devices, cosmetics, and detergents[29]. Although there are members of this group such as paraben triclosan and phthalates, the most well-known member is BPA.
In 1891, the first known EDC, BPA, was synthesized and was considered to be estrogenic in 1936 (Figure 1)[9]. Global production of BPA has increased progressively from 5 to 8 million tons (MT) between 2010 and 2016 and is estimated to reach 10.2 MT by 2022[30,31]. Most of the BPA produced is used to produce polycarbonates and epoxy resins[32,33]. However, it is readily found in air[34], soil[35], water[36], food[37], and in living organisms (humans[36], wildlife[33], and aquatic organisms[38]). In humans, BPA exposure occurs mainly through inhalation, ingestion, and dermal absorption[39]. The presence of BPA in toys, food packaging, and its resin in the lining of conserved food and beverages, to which virtually everyone is constantly exposed[40], facilitates exposure. In food contact materials, BPA may leach into food or water when exposed to high heat, repeated use, or physical manipulation. Studies on BPA have shown that BPA induces weight gain in mice and a high risk of T2DM[28]. Another study conducted in mice showed us that long-term BPA exposure plays a role in the development of glucose tolerance and decreased insulin secretion[41-43]. On the other hand, in humans, prenatal exposure has been shown to be associated with an increase in body fat by age 7 and an increase in body mass index by age 9[44]. These studies show us how closely BPA is related to diabetes and obesity.
As mentioned in the forthcoming sections, when various countries restricted or banned the use of BPA, BPA substitutes [e.g., bisphenol S (BPS) and bisphenol F (BPF) and bisphenol AF (BPAF)] began to be used instead of BPA (Figure 1). However, some later studies have revealed that these substitutes also present hormonal activity[45,46]. And these analogues are increasingly being detected in human urine[47,48]. The products labeled as “BPA free” use BPA analogues[46]. This situation also enlarges the risk of exposure to these chemicals in the intrauterine period or in infancy.
Phthalates are a group of chemicals used as liquid plasticizers to increase the elasticity of plastic products (Figure 1). Medical devices, including parenteral feeding tubes, personal care products such as nail polish and perfume, food packaging, and toys contain various phthalates[49]. Unfortunately, phthalates are poorly bio-degradable and highly bioaccumulative in the food chain[50]. It was found that the highest exposure comes from foods that absorb the compound from their packaging or during the production process[28]. Tordjman et al[51], notes that phthalates can accumulate in fatty foods such as mayonnaise, dairy products, fatty meat and fish, as well as shellfish, as they have a high solubility in fat. High phthalate exposure has been linked with increased threat of obesity and infertility, increased body mass index (BMI) and waist circumference, insulin resistance, and a change in thyroid hormones[49,52]. Many epidemiological studies have been conducted, especially on body weight and obesity. Although many of these studies have found an association between phthalates, obesity, and weight gain[53-55], some studies have not found a significant association[56]. On the contrary, a few studies have reported a negative correlation[57]. The correlation between phthalate exposure and obesity is, obviously controversial. It should be emphasized that most studies are cross-sectional. Therefore, large prospective studies are needed that would confirm or invalidate the existence of this association.
Triclosan is an extensively used antibacterial agent generally found in antibacterial soaps, toothpaste, oral rinses, toothbrushes, cutting boards, detergents, and plastics in furniture, toys, and sporting goods (Figure 1)[28,58]. Since Triclosan (TCS) can be mixed into nature from domestic wastewater, water and food can also be considered as exposure routes[59]. After ingestion, TCS can be noticed in the blood, plasma, milk, urine, brain, liver, and adipose tissue[60]. In the Weatherly and Gosse’s study[61], it was noted that TSC reduces the total level of T3[62], increases the risk of spontaneous abortion[63], reduces fertility[64], and lowers BMI[65]. Although this and many similar studies consider TCS to be a substance that threatens human health, another group has not been able to determine a correlation that would require TCS to be classified as EDC.
Polybrominated diphenyl ethers (PBDEs), commonly used in upholstered furniture, car seats, cushions, carpet padding and clothing, are mainly used as flame retardants (Figure 1)[44]. Humans may be exposed to polybrominated diphenyl ethers through consumption of foods such as fatty fish, consumption of contaminated water or soil, and inhalation of air containing polybrominated diphenyl ethers[66]. Due to their highly lipophilic structure, PBDEs tend to accumulate in adipose tissue and these chemicals can be released into the blood, especially during weight loss[8]. Many studies show that PBDEs are associated with metabolic syndrome and obesity[67,68]. In addition, insulin resistance in obese individuals has also been associated with the accumulation of PBDEs in adipose tissue[69]. After the phasing out of pentobromine diphenyl ethers in the United States and their prohibition in the European Union[70,71], alternative PBDEs were used in new furniture. However, it is suspected that these compounds may also affect hormonal activities[72,73].
The term obesogen was coined for chemicals that promote weight gain and obesity. Obesogens can promote obesity by increasing the number of adipocytes and fat storage in existing adipocytes, changing the calories burned at rest, changing the energy balance, and finally regulating appetite and satiety. In other words, obesogens can cause the development of obesity in a person's later life by modifying the "set point". It can be said that the main mechanism of the effects of obesogen exposure in later life during development is epigenetic changes. The individual's unique epigenetic system determines how the individual's hormonal regulatory system works. These decisions are, of course, directly related to hormonal processes such as appetite and weight gain. Epigenetic regulation of gene expression involves methylation of the CpG islands[74], probably in the gene promoter region, leading to repression of gene expression, and covalent modification of the tails of histone proteins that package DNA[75].
These modifications that occur during the development of the tissue are involved in the differentiation of the tissue during cell division, especially during mitosis. Although generally stable, environmental chemicals and changes in diet can lead to changes in the epigenetic marks, especially during development[8,75]. In conclusion, the obesity hypothesis makes two important points. The first is that the susceptibility to obesity begins during the development of the individual, both intrauterine and in the first years of life. The second is that EDCs that alter developmental programming, i.e., the “set point”, predispose one to weight gain and obesity later in life[14]. Another important conclusion is that habitual causes such as diet and exercise are not the only root of obesity, as we have seen in studies of obesity. It has also been shown that genes and environment (especially in the intrauterine and subsequent postpartum years), and the interactions between them are quite important.
In his article, Heindel et al[14] explains the importance of the obesogen hypothesis for the treatment of obesity and states that the obesogen hypothesis has shifted attention from genetics to the environment as a major cause of obesity. The focus of discussion has shifted from mostly unsuccessful treatment to prevention, and the timing of predisposition to obesity has shifted from adulthood to the developmental age. Therefore, it was helpful for us to focus on a different age group and the protection of that age group. Furthermore, this hypothesis gives us hope that the pandemic of obesity can be prevented by arguing that obesity is a disease acquired by environmental exposure, and therefore preventable, rather than a genetic inheritance.
The diabetogen hypothesis is similar and parallel to the obesity hypothesis. The main reason for this hypothesis is that some EDCs, especially POPs, predispose to T2DM independently of obesity[13]. Many studies have shown that POPs and others, increases peripheral insulin resistance and alters insulin production and secretion[13,76]. The diabetogenic effect of EDCs, at least partially, explains why some people are metabolically obese despite being of normal weight[77].
Due to ethical issues, it is quite difficult to design a double-blind trial on EDCs. For this reason, the studies can be divided into these four groups: Embryological analysis of EDC exposure (mainly in rodent models), studies of the mechanism of exposure (gene expression and epigenetic changes in cell and tissue cultures), studies examining the association between EDC and diseases (most of which are epidemiological studies), and reports of occupational or acute exposure to one or more chemicals known to be EDCs.
BPA is mainly used to make epoxy resins and polycarbonate. It is by far one of the most commonly exposed EDCs because of its widespread use and, particularly, its long-term use in sectors such as toys and food packaging. As a result, epidemiological studies have concentrated on BPA and phthalates in general. Only the most up-to-date studies and studies with divergent results are included here, because the study models are generally similar and there are no obvious differences between the outcomes.
Various recent studies have found a correlation between BPA and obesity. BPA urine levels were strongly correlated with BMI and waist circumference in 296 women of reproductive age[78]. In the study conducted by Choi et al[79], it measured the prenatal BPA exposures of 59 children. They classified those remaining above the 80th percentile as high exposure and others as low exposure. The study examined 594 obesity-associated CpG sites from children’s methylation profiles. As a result, high methylation in the insulin-like growth factor 2 receptor region was detected in the 2-year-old high exposure group. This study has strengthened the notion that BPA exposure affects humans through the mechanism of methylation[79].
Extensive studies published in recent years clearly reveal the relationship between BPA and obesity. A study of 888 middle-aged and elderly Chinese conducted in China found a positive association between urinary BPA concentration and central obesity[80]. A study by the Canadian Health Measures Survey found a positive correlation between urinary BPA levels and BMI-defined obesity. However, no relationship was found between waist circumference and urinary BPA using the standard cut-offs. In addition, for each natural logarithmic unit increase in urinary BPA concentration, an increase in the BMI of 0.33 kg/m2 and a waist circumference of 1 cm was observed[81]. The National Health and Nutrition Examination Survey (NHANES) studies in 2003-04 and 2005-06 found a significant positive association with obesity when comparing the highest and lowest quartiles of urinary BPA[82]. This study was adjusted for traditional risk factors. Likewise, the Korean national health study used the Covariate-Adjusted Standardization (CAS) Method to prevent factors such as age, gender, education, urinary creatinine, income, alcohol, and smoking from affecting the study. After using the CAS method, a positive relationship with obesity was determined by comparing the highest and lowest quartiles of urinary BPA[83]. Wu et al[84] showed in their meta-analysis that there was a positive correlation between BPA exposure and obesity. According to the dose-response analysis, an increase of 1 ng/mL BPA causes an increase of 11% risk in obesity.
Evidence from individual epidemiological studies is usually inconsistent in regards to the association of BPA exposure and T2DM, so it is unclear whether exposure to BPA is a risk factor for the development of T2DM. Population ethnicity, lifestyle, eating habits, use of BPA-containing products, different sample size, and differences in T2DM criteria are the factors that may account for the inconsistent findings[85]. One of the meta-analysis (n = 41.320) included 16 epidemiological studies (12 cross-sectional, 2 case-control, 1 prospective) that showed a positive association between BPA levels and T2DM risk with a pooled OR of 1.28 (95%CI 1.14-1.44)[86]. Another meta-analysis, including 41 cross-sectional and 8 prospective studies from ethnically diverse populations, evaluated the association of dioxin, PCB, chlorinated pesticide, BPA and phthalate with T2DM, and related metabolic features. Serum concentrations of dioxins, PCBs, and chlorinated pesticides were significantly associated with T2DM risk. Urinary concentrations of BPA and phthalates were also associated with T2DM risk with pooled RR of 1.45 (95%CI 1.13-1.87) and 1.48 (95%CI 0.98-2.25), respectively[87].
On the other hand, few studies have shown that BPA can trigger diabetes independent of obesity. Silver et al[88] analyzed the NHANES results and Tai et al[89] analyzed the CMHS results and found a positive correlation between BPA and glycated haemoglobin. Another study reversed the experimental mechanism. When the study compared urinary BPA in individuals with impaired glucose tolerance (IGT) and normal glucose tolerance (NGT), the results showed that urinary BPA was higher in individuals with IGT[90]. When a similar study was conducted with participants with T2DM and NGT, it was found that participants with T2DM had higher urinary BPA[91]. In addition, BPA has also been found to be positively associated with high blood insulin resistance[76,87], prediabetes risk[92], and T2DM[93,94].
Many countries have regulated BPA. BPA was banned from bottles in Canada in 2008, Denmark in 2009, France in 2010, and the European Union and Turkey in 2011[95-97]. In addition, Sweden and France banned the use of BPA in food products designed for children under the age of 3 in 2011 and 2013, respectively[96]. Such regulations have led to the development of substitutes such as BPS and BPF.
However, as previously mentioned, the hormonal activities of BPA substitutes (generally BPS, BPF, BPAF) were also of the same magnitude and similar effect as BPA in the in vivo and in vitro studies[46]. A NHANES analysis showed the positive association between BPS and obesity, particularly in children and adolescents[98,99]. When Duan et al[100] examined BPA equivalents BPAF and BPS, they reported a positive correlation with T2DM.
A group analyzed the NHANES 2003-2014 results. They state that the urinary BPA concentration has decreased over the years in a trend analysis. Bans on BPA since 2009 may have contributed to this. As a result, the researchers indicated that the positive relationship between obesity and urinary BPA diminished between 2009 and 2014[101]. Since then however, obesity has been on the rise. It is noteworthy that the oral bioavailability of EDC candidate chemicals such as BPS is higher than BPA[102] and that the half-life is longer[103].
EDCs can mimic hormonal actions through the nuclear hormone receptor superfamily like steroid hormone receptors, thyroid hormone receptors, retinoid X receptors (RXR), peroxisome proliferator-activated receptors (PPAR), liver X receptors, and farnesoid X receptors[104]. The nuclear hormone receptor PPAR gamma dimerize with RXR and bind to PPAR-responsive DNA regulatory elements, which leads to the controlling of fat cell differentiation. In this way, genes responsible for adipogenesis, lipid, and glucose metabolism are controlled[105]. Dysregulation of PPARγ can cause obesity and metabolic disorders. PPARγ can bind to BPA, perfluorinated compounds (PFCs) and phthalates leading to stimulation of adipogenesis in vitro and in vivo by stimulating the differentiation of preadipocytes to mature adipocytes[105]. The differentiation of adipocytes is a complex process. The expression of binding proteins CCAAT/enhancer beta (C/EBPß) and C/EBPδ induces adipogenesis firstly. At the second stage, the activity of those proteins leads to the activation of PPARγ and C/EBPα[106]. There are different models of cell lines which are embryonic 3T3-L1 and 3T3-F442A cell lines that can be stimulated to differentiate into adipocytes under chemical exposure[28].
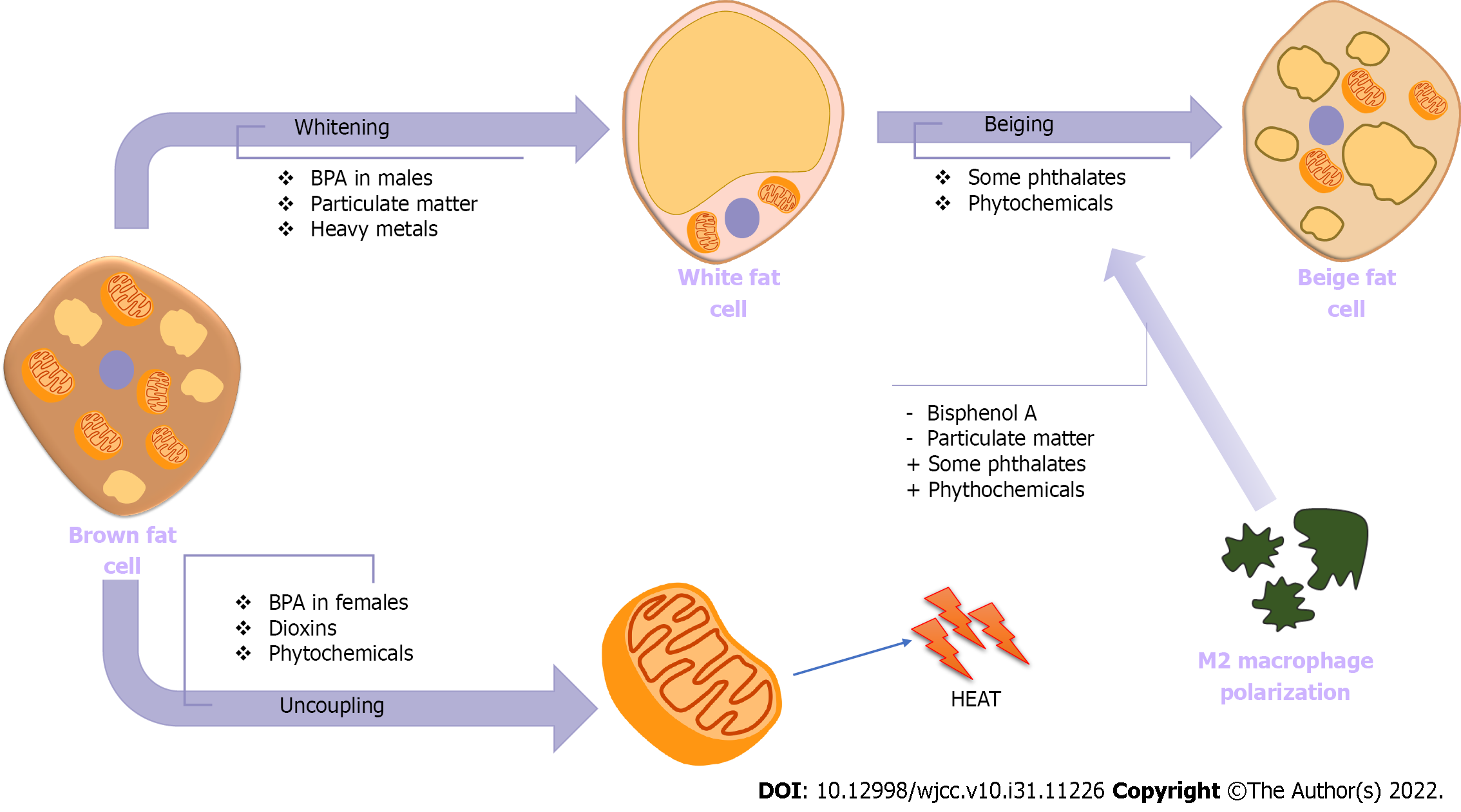
White adipose tissue (WAT) is the main adipose tissue for the fat storage. But specific adipose tissue like brown adipose tissue (BAT) and beige fat are also present. Those have thermogenic features primarily mediated by the expression of uncoupling protein 1 (UCP1). BAT is activated at birth for thermogenesis, but with increasing age, this activity of BAT decreases. Beige fat differentiates from white adipocytes postnatally in response to cold temperatures, thyroid hormone, adipokines, immune cells, and cytokines. Those two thermogenic adipose tissues have dense mitochondria and UCP1 Leading to increased basal metabolic rate, and metabolizing excess fat as anti-obesity features. The loss of BAT called as whitening, and decreases in the beiging of white adipose tissue, are associated with aging, obesity, and metabolic diseases[107]. Transcriptional regulation of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC1α), and UCP1 is performed by thyroid receptors and PPARs. EDCs interfere with thyroid or PPAR signalling, so in that way, EDCs can cause dysfunction in brown and beige fat depots (Figure 2). Female offspring from dams exposed to BPA had an increment in weight of the interscapular BAT depot and the expression of UCP1[108]. However, male offspring demonstrated reduced brown adipogenesis and BAT activity. An agonistic effect of BPA on the estrogen receptor, which is necessary for mitochondrial biogenesis and thermogenesis in BAT, can explain the sexual dimorphism observed with BPA[109].
The other potential explanation of BPA effect on WAT and BAT is the distribution of macrophage subtypes. When M1 macrophages are activated, WAT inflammation occurs through secretion of proinflammatory cytokines including interleukin-1ß (IL-1ß), IL-6, and tumor necrosis factor-α (TNF-α). M2 macrophages secrete anti-inflammatory cytokines such as IL-4 and IL-10[110]. M1 macrophage infiltration accompanies whitening of brown fat, suggestive of BAT dysfunction (Figure 2). Some phytochemicals and phthalates also can promote M2 macrophage polarization and beiging in WAT[111,112].
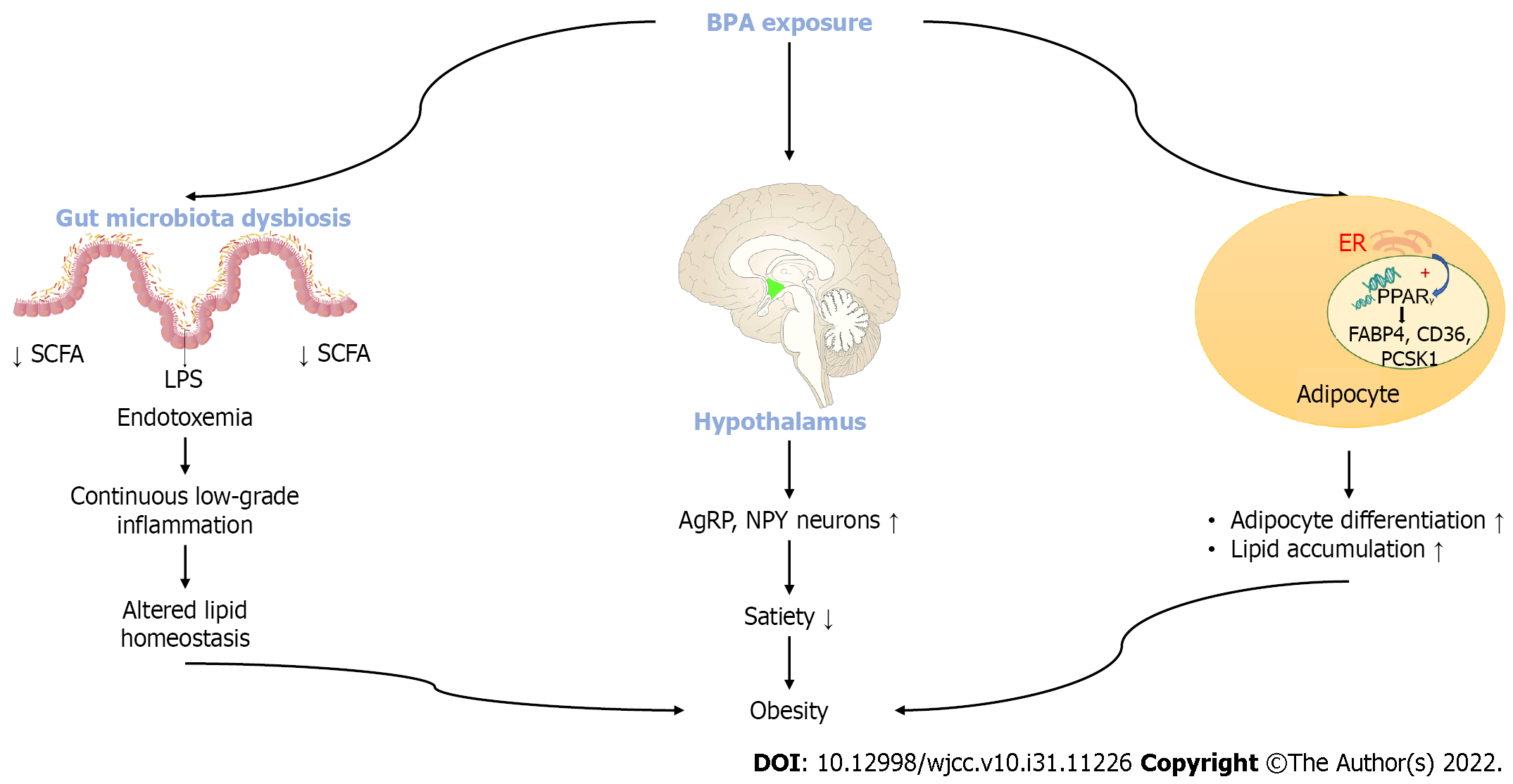
BPA prevents the release of adiponectin while it increases pro-inflammatory cytokines by acting on adipocytes and macrophages. BPA also regulates some genes like fatty acid binding protein 4 (FABP4) and a cluster of differentiation 36 (CD36). FABP4 binds to lipid A resulting in accumulation of fatty acids. Up-regulation of FABP4 in adipose tissue prevents fatty acid ß oxidation. Increased fatty acid levels go to the ectopic organs such as the liver resulting in lipotoxicity[113]. Besides a lipid accumulation effect of BPA leading to obesity, two other mechanisms to explain obesity due to BPA exposure is defined (Figure 3). BPA can result in an interruption of the neuroendocrine system in the central nervous system. Exposure to BPA can stimulate the release of Agouti related peptide (AgRP) and neuropeptide Y (NPY) and can decrease the level of proopiomelanocortin (POMC). AgRP and NPY are the orexigenic hormones inducing appetite. BPA also can have an effect on gut bacterial dysbiosis. BPA reduces gut small chain fatty acid (SCFA) and increases systemic lipopolysaccharide levels resulting in chronic low-grade inflammation and altered lipid homeostasis[113] (Figure 3).
The studies provide a strong support for an association between BPA, ß cell function and insulin resistance[85]. In rat insulinoma cell lines, BPA decreases cell viability, disrupts glucose stimulated insulin secretion (GSIS), and induces apoptosis in a dose-dependent manner. BPA causes an increased expression of pro-apoptotic Bax protein and a reduced expression of anti-apoptotic Bcl-2[114]. It was shown that ß cell damage due to BPA can be through the interaction of BPA with human islet amyloid polypeptide (hIAPP) leading to islet amyloid aggregate formation[115].
Alonso-Magdalena et al[116] demonstrated that acute exposure to BPA induces a rapid decrease in the glycemia within 30 min of the first injection by increasing in plasma insulin. Sustained exposure to BPA resulted in higher ß-cell insulin levels due to estrogen receptor stimulation. Both the estrogen and the BPA treated mice showed 1.7- and 1.53-fold higher circulating insulin levels, respectively with normal blood glucose levels mimicking insulin resistance features. BPA has a non-monotonic dose-response effect, which means the most effective dose may not necessarily be the highest one. There is an inverted U-shaped relationship between increasing BPA doses and GSIS. While a dose of 0.1 µg/L BPA causes an increase in GSIS, doses of 25 and 250 µg/L BPA cause a decrease in GSIS[117,118].
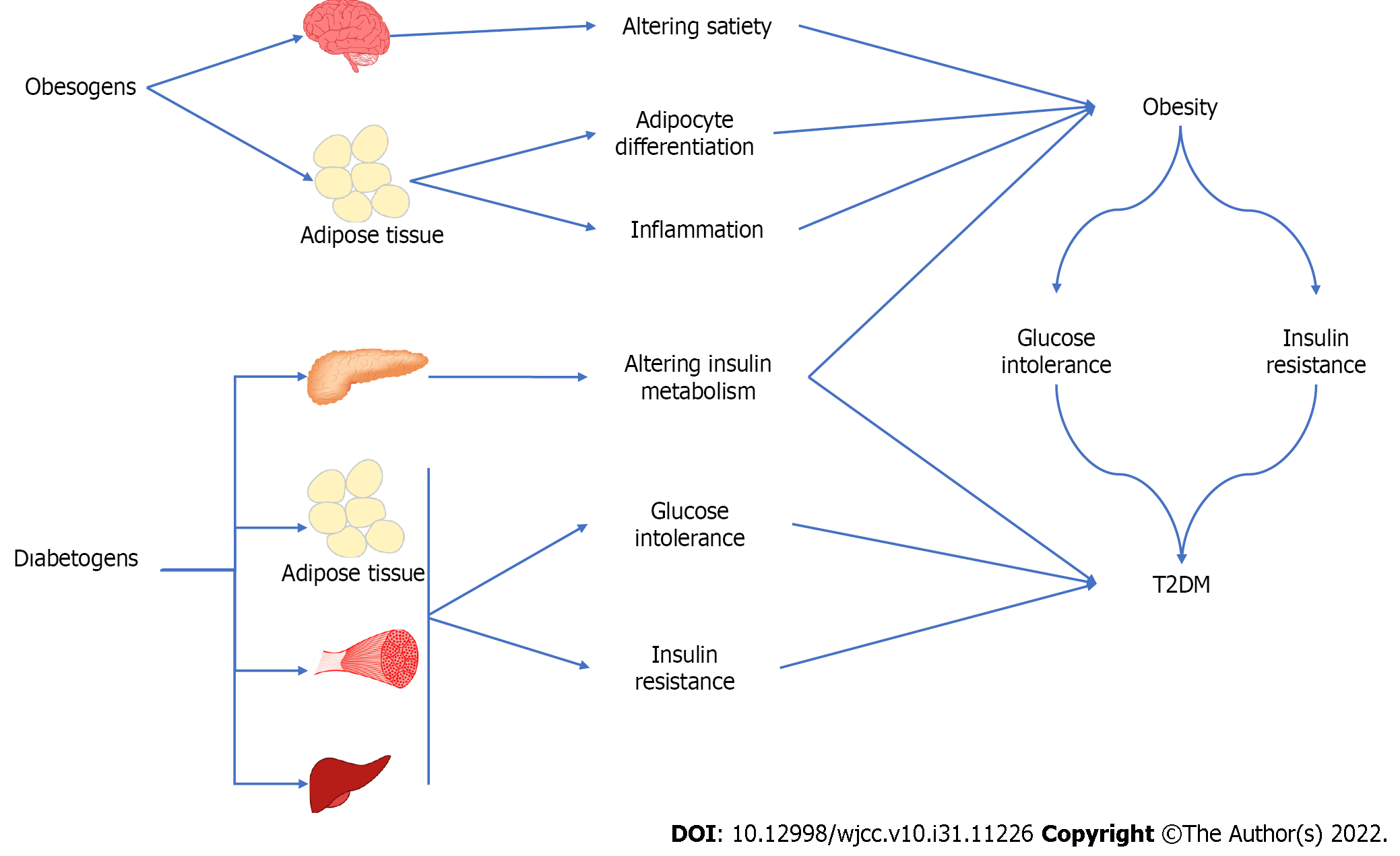
The effect of EDCs as obesogens and diabetogens causing obesity and T2DM is summarized in Figure 4.
Humans are exposed to a variety of EDCs with obesogenic and diabetogenic effects. Those chemicals change the hormonal balance with different mechanisms of action. The exposure to obesogens can happen during the different stages of life. In this context, perinatal exposure can be important as far as the permanent and transgenerational effects are concerned. EDCs promote adipogenesis leading to fat accumulation, which causes alteration in lipid metabolism and satiety as obesogens. EDCs have shown the potential to induce adipose tissue dysfunction not only in white adipocytes but in brown and beige fat as well. Besides an obesogenic effect, EDCs can cause T2DM through alteration in ß cell function and morphology, and insulin resistance. The studies related to EDCs and obesity, and T2DM are not enough methodologically to show causality. In this respect, randomized clinical trials are needed to investigate the association between obesogen, diabetogen, and the related metabolic clinical picture. Future studies will be important to take political action and to increase the awareness of the population about the exposure to obesogen and diabetogen EDCs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Endocrine Society, No. 598888.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen K, China; Srinivasan AR, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3869] [Cited by in F6Publishing: 4280] [Article Influence: 611.4] [Reference Citation Analysis (2)] |
| 2. | World Health Organization. World Obesity Atlas 2022. [cited 30 April 2022]. Available from: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022. [Cited in This Article: ] |
| 3. | World Health Organization. International Classification of Diseases 11th Revision. [cited 30 April 2022]. Available from: https://icd.who.int/en. [Cited in This Article: ] |
| 4. | Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1741] [Cited by in F6Publishing: 2234] [Article Influence: 446.8] [Reference Citation Analysis (0)] |
| 5. | International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: International Diabetes Federation; 2021. [Cited in This Article: ] |
| 6. | World Health Organization. Global report on diabetes. April 21, 2016. [cited 30 April 2022]. Available from: https://www.who.int/publications-detail-redirect/9789241565257. [Cited in This Article: ] |
| 7. | World Health Organization. Diabetes. May 5, 2022. [cited 30 April 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. [Cited in This Article: ] |
| 8. | Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36:E1-E150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1466] [Cited by in F6Publishing: 1233] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 9. | Dodds EC, Lawson W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature. 1936;137:996. [DOI] [Cited in This Article: ] [Cited by in Crossref: 422] [Cited by in F6Publishing: 421] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Ghosh S, Murinova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, Dutta SK. Biomarkers linking PCB exposure and obesity. Curr Pharm Biotechnol. 2014;15:1058-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. Food safety: persistent organic pollutants (POPs). November 2, 2020. [cited 30 April 2022]. Available from: https://www.who.int/news-room/questions-and-answers/item/food-safety-persistent-organic-pollutants-(pops). [Cited in This Article: ] |
| 12. | Jones KC, de Voogt P. Persistent organic pollutants (POPs): state of the science. Environ Pollut. 1999;100:209-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1025] [Cited by in F6Publishing: 782] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 13. | La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect. 2013;121:162-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 14. | Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 673] [Cited by in F6Publishing: 622] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 15. | Can-Güven E, Gedik K, Kurt-Karakuş PB. Organochlorine pesticides and polychlorinated biphenyls from a greenhouse area on the Mediterranean coast of Turkey: Distribution, air-soil exchange, enantiomeric signature, and source implications. Atmos Pollut Res. 2022;13:101263. [Cited in This Article: ] |
| 16. | Kurt-Karakus PB, Ugranli-Cicek T, Sofuoglu SC. The first countrywide monitoring of selected POPs: Polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and selected organochlorine pesticides (OCPs) in the atmosphere of Turkey. Atmos Environ. 2018;177:154-165. [DOI] [Cited in This Article: ] |
| 17. | Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 397] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 18. | Song W, Chen S, Liu J. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agric. 2015;14:1845-1854. [DOI] [Cited in This Article: ] |
| 19. | Adil MF, Sehar S, Chen G, Chen ZH, Jilani G, Chaudhry AN, Shamsi IH. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological / ultrastructural adjustments. Ecotoxicol Environ Saf. 2020;190:110076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, Gottardis MM, Solomon HB, Sholler PF, Jordan VC, Martin MB. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem. 1994;269:16896-16901. [PubMed] [Cited in This Article: ] |
| 21. | Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14:545-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Doolan G, Benke G, Giles G. An update on occupation and prostate cancer. Asian Pac J Cancer Prev. 2014;15:501-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci. 2007;98:75-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Davey JC, Nomikos AP, Wungjiranirun M, Sherman JR, Ingram L, Batki C, Lariviere JP, Hamilton JW. Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis. Environ Health Perspect. 2008;116:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Yang CY, Chang CC, Chiu HF. Does arsenic exposure increase the risk for prostate cancer? J Toxicol Environ Health A. 2008;71:1559-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Díaz-Villaseñor A, Sánchez-Soto MC, Cebrián ME, Ostrosky-Wegman P, Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic beta-cells. Toxicol Appl Pharmacol. 2006;214:30-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol. 2013;267:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | González-Casanova JE, Pertuz-Cruz SL, Caicedo-Ortega NH, Rojas-Gomez DM. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. Biomed Res Int. 2020;2020:7453786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Cho YJ, Yun JH, Kim SJ, Kwon HY. Nonpersistent endocrine disrupting chemicals and reproductive health of women. Obstet Gynecol Sci. 2020;63:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Reportlinker. Bisphenol-A–A Global Market Overview. [cited 22 June 2022]. Available from: https://www.reportlinker.com/p03670134/Bisphenol-A-A-Global-Market-Overview.html. [Cited in This Article: ] |
| 31. | Staples C, van der Hoeven N, Clark K, Mihaich E, Woelz J, Hentges S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere. 2018;201:448-458. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 533] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 33. | Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response. 2015;13:1559325815598308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 388] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 34. | Hines CJ, Jackson MV, Christianson AL, Clark JC, Arnold JE, Pretty JR, Deddens JA. Air, hand wipe, and surface wipe sampling for Bisphenol A (BPA) among workers in industries that manufacture and use BPA in the United States. J Occup Environ Hyg. 2017;14:882-897. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Chakraborty P, Sampath S, Mukhopadhyay M, Selvaraj S, Bharat GK, Nizzetto L. Baseline investigation on plasticizers, bisphenol A, polycyclic aromatic hydrocarbons and heavy metals in the surface soil of the informal electronic waste recycling workshops and nearby open dumpsites in Indian metropolitan cities. Environ Pollut. 2019;248:1036-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Santhi VA, Sakai N, Ahmad ED, Mustafa AM. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci Total Environ. 2012;427-428:332-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24:139-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1904] [Cited by in F6Publishing: 1871] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 38. | Canesi L, Fabbri E. Environmental Effects of BPA: Focus on Aquatic Species. Dose Response. 2015;13:1559325815598304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | Li D, Suh S. Health risks of chemicals in consumer products: A review. Environ Int. 2019;123:580-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect. 2011;119:1396-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | García-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;9:e100214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Moon MK, Jeong IK, Jung Oh T, Ahn HY, Kim HH, Park YJ, Jang HC, Park KS. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J Endocrinol. 2015;226:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61:1495-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Wong KH, Durrani TS. Exposures to Endocrine Disrupting Chemicals in Consumer Products-A Guide for Pediatricians. Curr Probl Pediatr Adolesc Health Care. 2017;47:107-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Reina-Pérez I, Olivas-Martínez A, Mustieles V, Ruiz-Ojeda FJ, Molina-Molina JM, Olea N, Fernández MF. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem Toxicol. 2021;152:112216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123:643-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 898] [Cited by in F6Publishing: 910] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Guan J, Yin J, Shao B, Li H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere. 2014;112:481-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000-2014. Environ Sci Technol. 2015;49:11834-11839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 49. | Arrebola JP, Alzaga BG. Exposición a contaminantes ambientales por vía alimentaria y repercusiones metabólicas relacionadas con la obesidad. Nut Clin Med. 2016;10:164-174. [DOI] [Cited in This Article: ] |
| 50. | Mezcua M, Martínez-Uroz MA, Gómez-Ramos MM, Gómez MJ, Navas JM, Fernández-Alba AR. Analysis of synthetic endocrine-disrupting chemicals in food: a review. Talanta. 2012;100:90-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Tordjman K, Grinshpan L, Novack L, Göen T, Segev D, Beacher L, Stern N, Berman T. Exposure to endocrine disrupting chemicals among residents of a rural vegetarian/vegan community. Environ Int. 2016;97:68-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Srivastava V, Srivastava T, Kumar MS. Fate of the persistent organic pollutant (POP) Hexachlorocyclohexane (HCH) and remediation challenges. Int Biodeterior Biodegradation. 2019;140:43-56. [DOI] [Cited in This Article: ] |
| 53. | Amin MM, Ebrahimpour K, Parastar S, Shoshtari-Yeganeh B, Hashemi M, Mansourian M, Poursafa P, Fallah Z, Rafiei N, Kelishadi R. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 55. | Kim JH, Park H, Lee J, Cho G, Choi S, Choi G, Kim SY, Eun SH, Suh E, Kim SK, Kim HJ, Kim GH, Lee JJ, Kim YD, Eom S, Kim S, Moon HB, Park J, Choi K. Association of diethylhexyl phthalate with obesity-related markers and body mass change from birth to 3 months of age. J Epidemiol Community Health. 2016;70:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Lim JE, Choi B, Jee SH. Urinary bisphenol A, phthalate metabolites, and obesity: do gender and menopausal status matter? Environ Sci Pollut Res Int. 2020;27:34300-34310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, Silva MJ, Brenner BL, Wolff MS. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 58. | Zamkowska D, Karwacka A, Jurewicz J, Radwan M. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int J Occup Med Environ Health. 2018;31:377-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, Surampalli RY. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015;12:5657-5684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 60. | Wang CF, Tian Y. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environ Pollut. 2015;206:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 61. | Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev. 2017;20:447-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 62. | Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007-2008. Sci Total Environ. 2013;445-446:299-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 63. | Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Chen L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Sci Rep. 2015;5:18252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 64. | Vélez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril. 2015;103:1011-1020.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 65. | Li S, Zhao J, Wang G, Zhu Y, Rabito F, Krousel-Wood M, Chen W, Whelton PK. Urinary triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: Experience in NHANES 2003-2010. Int J Hyg Environ Health. 2015;218:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Environmental Protection Agency Federal Facilities Restoration U, Office R. Technical Fact Sheet-Polybrominated Diphenyl Ethers (PBDEs). 2017. [Cited in This Article: ] |
| 67. | Yang C, Kong APS, Cai Z, Chung ACK. Persistent Organic Pollutants as Risk Factors for Obesity and Diabetes. Curr Diab Rep. 2017;17:132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Lim JS, Lee DH, Jacobs DR Jr. Association of brominated flame retardants with diabetes and metabolic syndrome in the U.S. population, 2003-2004. Diabetes Care. 2008;31:1802-1807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Helaleh M, Diboun I, Al-Tamimi N, Al-Sulaiti H, Al-Emadi M, Madani A, Mazloum NA, Latiff A, Elrayess MA. Association of polybrominated diphenyl ethers in two fat compartments with increased risk of insulin resistance in obese individuals. Chemosphere. 2018;209:268-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46:13432-13439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 71. | News-ECHA. SEAC concludes on Bisphenol A, DecaBDE and PFOA restrictions and finalises two opinions for authorisation [Cited 22 June 2022]. Available from: https://echa.europa.eu/view-article/-/journal_content/title/seac-concludes-on-bisphenol-a-decabde-and-pfoa-restrictions-and-finalises-two-opinions-for-authorisation. [Cited in This Article: ] |
| 72. | Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 73. | Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro. Environ Health Perspect. 2014;122:1225-1232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 75. | Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62:1789-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 76. | Beydoun HA, Khanal S, Zonderman AB, Beydoun MA. Sex differences in the association of urinary bisphenol-A concentration with selected indices of glucose homeostasis among U.S. adults. Ann Epidemiol. 2014;24:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 77. | Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 560] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 78. | Hong SH, Sung YA, Hong YS, Ha E, Jeong K, Chung H, Lee H. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin Endocrinol (Oxf). 2017;86:506-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Choi YJ, Lee YA, Hong YC, Cho J, Lee KS, Shin CH, Kim BN, Kim JI, Park SJ, Bisgaard H, Bønnelykke K, Lim YH. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ Int. 2020;143:105929. [PubMed] [Cited in This Article: ] |
| 80. | Hao M, Ding L, Xuan L, Wang T, Li M, Zhao Z, Lu J, Xu Y, Chen Y, Wang W, Bi Y, Xu M, Ning G. Urinary bisphenol A concentration and the risk of central obesity in Chinese adults: A prospective study. J Diabetes. 2018;10:442-448. [PubMed] [Cited in This Article: ] |
| 81. | Do MT, Chang VC, Mendez MA, de Groh M. Urinary bisphenol A and obesity in adults: results from the Canadian Health Measures Survey. Health Promot Chronic Dis Prev Can. 2017;37:403-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 82. | Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1283] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 83. | Lee I, Park YJ, Kim MJ, Kim S, Choi S, Park J, Cho YH, Hong S, Yoo J, Park H, Cheon GJ, Choi K, Moon MK. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015-2017. Environ Int. 2021;146:106227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 84. | Wu W, Li M, Liu A, Wu C, Li D, Deng Q, Zhang B, Du J, Gao X, Hong Y. Bisphenol A and the Risk of Obesity a Systematic Review With Meta-Analysis of the Epidemiological Evidence. Dose Response. 2020;18:1559325820916949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 85. | Farrugia F, Aquilina A, Vassallo J, Pace NP. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int J Environ Res Public Health. 2021;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 86. | Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord. 2018;18:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 87. | Song Y, Chou EL, Baecker A, You NC, Song Y, Sun Q, Liu S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J Diabetes. 2016;8:516-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 88. | Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. 2011;6:e26868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Tai X, Chen Y. Urinary bisphenol A concentrations positively associated with glycated hemoglobin and other indicators of diabetes in Canadian men. Environ Res. 2016;147:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Chailurkit LO, Tengpraettanakorn P, Chanprasertyotin S, Ongphiphadhanakul B. Is bisphenol A exposure associated with the development of glucose intolerance and increased insulin resistance in Thais? Nutr Health. 2017;23:185-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Soundararajan A, Prabu P, Mohan V, Gibert Y, Balasubramanyam M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol Cell Biochem. 2019;458:171-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50:625-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 93. | Rancière F, Botton J, Slama R, Lacroix MZ, Debrauwer L, Charles MA, Roussel R, Balkau B, Magliano DJ; D. E.S.I.R. Study Group. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case-Cohort Study in the French Cohort D.E.S.I.R. Environ Health Perspect. 2019;127:107013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 94. | Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822-3826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 95. | Moon MK. Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab J. 2019;43:46-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 96. | Detailed Pedia. Health effects of Bisphenol A-Wikipedia. [cited 23 May 2022]. Available from: https://en.wikipedia.org/wiki/Health_effects_of_Bisphenol_A#Chemical_manufacturers_reactions_to_bans. [Cited in This Article: ] |
| 97. | Resmî Gazete. Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü. [cited 23 May 2022]. Available from: https://www.resmigazete.gov.tr/eskiler/2011/06/20110610-8.htm. [Cited in This Article: ] |
| 98. | Jacobson MH, Woodward M, Bao W, Liu B, Trasande L. Urinary Bisphenols and Obesity Prevalence Among U.S. Children and Adolescents. J Endocr Soc. 2019;3:1715-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 99. | Liu B, Lehmler HJ, Sun Y, Xu G, Sun Q, Snetselaar LG, Wallace RB, Bao W. Association of Bisphenol A and Its Substitutes, Bisphenol F and Bisphenol S, with Obesity in United States Children and Adolescents. Diabetes Metab J. 2019;43:59-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 100. | Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, Chen L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: A case-control study. Environ Pollut. 2018;243:1719-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 101. | Okubo Y, Handa A, Belin T. Serial cross-sectional study for the association between urinary bisphenol A and paediatric obesity: Recent updates using NHANES 2003-2014. Pediatr Obes. 2019;14:e12566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Gayrard V, Lacroix MZ, Grandin FC, Collet SH, Mila H, Viguié C, Gély CA, Rabozzi B, Bouchard M, Léandri R, Toutain PL, Picard-Hagen N. Oral Systemic Bioavailability of Bisphenol A and Bisphenol S in Pigs. Environ Health Perspect. 2019;127:77005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Gayrard V, Lacroix MZ, Gély CA, Grandin FC, Léandri R, Bouchard M, Roques B, Toutain PL, Picard-Hagen N. Toxicokinetics of bisphenol S in rats for predicting human bisphenol S clearance from allometric scaling. Toxicol Appl Pharmacol. 2020;386:114845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8:161-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 105. | Francis CE, Allee L, Nguyen H, Grindstaff RD, Miller CN, Rayalam S. Endocrine disrupting chemicals: Friend or foe to brown and beige adipose tissue? Toxicology. 2021;463:152972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1451] [Cited by in F6Publishing: 1538] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 107. | Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1542] [Cited by in F6Publishing: 1639] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 108. | van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, van der Ven LT. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology. 2014;321:40-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Zhou Z, Moore TM, Drew BG, Ribas V, Wanagat J, Civelek M, Segawa M, Wolf DM, Norheim F, Seldin MM, Strumwasser AR, Whitney KA, Lester E, Reddish BR, Vergnes L, Reue K, Rajbhandari P, Tontonoz P, Lee J, Mahata SK, Hewitt SC, Shirihai O, Gastonbury C, Small KS, Laakso M, Jensen J, Lee S, Drevon CA, Korach KS, Lusis AJ, Hevener AL. Estrogen receptor α controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci Transl Med. 2020;12. [PubMed] [Cited in This Article: ] |
| 110. | Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 920] [Cited by in F6Publishing: 972] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 111. | Miller GD. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am J Lifestyle Med. 2019;13:586-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Yen FS, Wang HC, Pan CW, Wei JC, Hsu CC, Hwu CM. Pioglitazone Exposure Reduced the Risk of All-Cause Mortality in Insulin-Treated Patients with Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Naomi R, Yazid MD, Bahari H, Keong YY, Rajandram R, Embong H, Teoh SH, Halim S, Othman F. Bisphenol A (BPA) Leading to Obesity and Cardiovascular Complications: A Compilation of Current In Vivo Study. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 114. | Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013;4:e460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 115. | Gong H, Zhang X, Cheng B, Sun Y, Li C, Li T, Zheng L, Huang K. Bisphenol A accelerates toxic amyloid formation of human islet amyloid polypeptide: a possible link between bisphenol A exposure and type 2 diabetes. PLoS One. 2013;8:e54198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 116. | Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 427] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 117. | Song L, Xia W, Zhou Z, Li Y, Lin Y, Wei J, Wei Z, Xu B, Shen J, Li W, Xu S. Low-level phenolic estrogen pollutants impair islet morphology and β-cell function in isolated rat islets. J Endocrinol. 2012;215:303-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Makaji E, Raha S, Wade MG, Holloway AC. Effect of environmental contaminants on Beta cell function. Int J Toxicol. 2011;30:410-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |