Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11044
Peer-review started: May 6, 2022
First decision: July 29, 2022
Revised: August 9, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: October 26, 2022
Posterior reversible encephalopathy syndrome (PRES) is a neuroimaging-based syndrome and is associated with multifocal vasogenic cerebral edema. Patients with PRES frequently demonstrate headache, seizure, encephalopathy, altered mental function, visual loss and so on. We here report a patient who showed persistent neurologic deficits after PRES and was ultimately diagnosed with autoimmune encephalitis (AE).
This case exhibits a rare imaging manifestation of anti-casper 2 encephalitis which was initially well-matched with PRES and associated vasogenic edema.
AE should be further considered when the etiology, clinical manifestations, and course of PRES are atypical.
Core Tip: Posterior reversible encephalopathy syndrome (PRES) is associated with many diverse clinical comorbid, the most common of which are hypertension, eclampsia, renal failure and immunosuppressive treatment. PRES is a neuroimaging-based syndrome and is associated with multifocal vasogenic cerebral edema. Patients with PRES are frequently manifested by headache, seizure, encephalopathy, altered mental function, visual loss, etc. We here report a patient who showed persistent neurologic deficits after PRES and was ultimately diagnosed with autoimmune encephalitis.
- Citation: Dai SJ, Yu QJ, Zhu XY, Shang QZ, Qu JB, Ai QL. Autoimmune encephalitis with posterior reversible encephalopathy syndrome: A case report. World J Clin Cases 2022; 10(30): 11044-11048
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11044.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11044
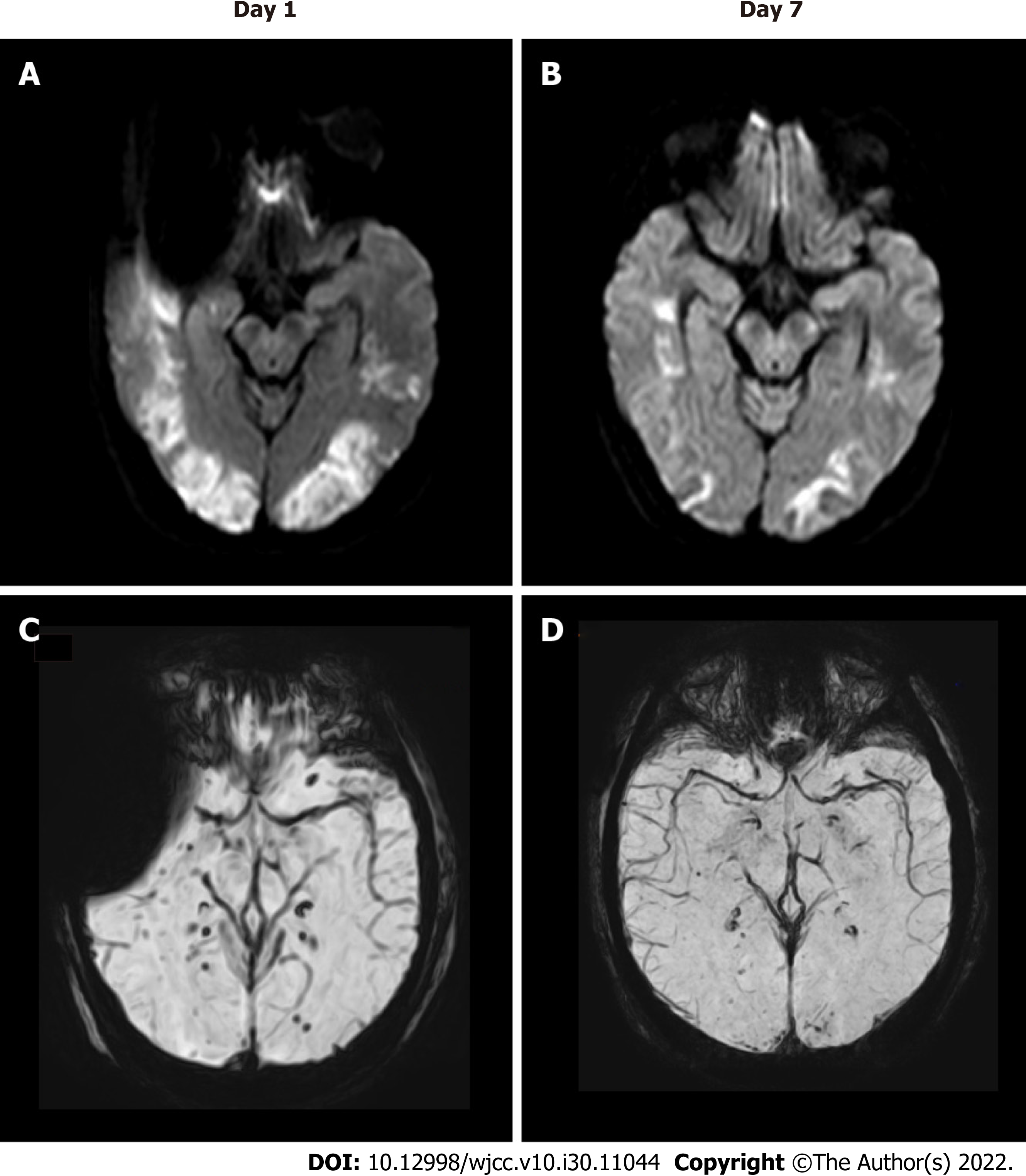
A 37-year-old man visited the Department of neurological care unit in the First Affiliated Hospital of Kunming Medical University complaining of a general fatigue for 1 wk, aggravated with disturbance of consciousness for 1 d. He had no past medical history. When admitted to the hospital, his Glasgow Coma Scale (GCS) score was 9 (E4/V1/M4), he showed a normal blood pressure, no cervical resistance, increased muscle tone, hyperreflexia and hyperactivity ankle clonus. At presentation, brain magnetic resonance imaging (MRI) showed bilateral multifocal vasogenic edema especially in his bilateral occipital lobes, which is compatible with posterior reversible encephalopathy syndrome (PRES) (Figure 1A). Spot-like microbleeds were found on susceptibility weighted imaging (SWI) mapping (Figure 1C). During admission, follow-up MRI at 1 wk showed reduced vasogenic edema in both cerebral hemispheres considerably, with less prominent microbleeds on SWI (Figure 1B and D). Serum and cerebrospinal fluid (CSF) autoantibody tests using a cell-based immunocytochemistry kit (Shaanxi MYBiotech Co., Ltd.) showed the presence of anti-Casper 2 antibody with tilter of 1: 3.2 in CSF and 1: 100 in blood serum. The patient’s CSF profile was otherwise normal (red blood cell 20/μL; white blood cell 1/μL; protein 0.25 g/L; and glucose 4.5 mmol/L) with no evidence of infection. The patient finally diagnosed as an anti-Casper 2 autoimmune encephalitis (AE). After intravenous immunoglobulin (IVIG) for 5 d (400 mg/kg/d), his GCS score increased to 10 (E4/V1/M5), which means patient’s movement was improved. Unfortunately, due to economic problem, he didn’t perform electroencephalography (EEG) and electromyography (EMG). After 3 wk, he was transferred to local hospital.
A general fatigue for 1 wk, aggravated with disturbance of consciousness for 1 d.
There is no special illness except the chief complaints.
There is no history of past illness.
There is no personal and family history.
The patient's GCS score was 9 (E4/V1/M4), he showed a normal blood pressure, no cervical resistance, increased muscle tone, hyperreflexia and hyperactivity ankle clonus.
Serum and CSF autoantibody tests using a cell-based immunocytochemistry kit (Shaanxi MYBiotech Co., Ltd.) showed the presence of anti-Casper 2 antibody with tilter of 1: 3.2 in CSF and 1: 100 in blood serum. The patient’s CSF profile was otherwise normal (red blood cell 20/μL; white blood cell 1/μL; protein 0.25 g/L; and glucose 4.5 mmol/L) with no evidence of infection.
Brain MRI showed bilateral multifocal vasogenic edema especially in his bilateral occipital lobes, which is compatible with PRES (Figure 1A). Spot-like microbleeds were found on SWI mapping (Figure 1C). During admission, follow-up MRI at 1 wk showed reduced vasogenic edema in both cerebral hemispheres considerably, with less prominent microbleeds on SWI (Figure 1B and D).
Anti-Casper 2 AE.
IVIG for 5 d (400 mg/kg/d).
After intravenous immunoglobulin (IVIG) for 5 d (400 mg/kg/d), his GCS score increased to 10 (E4/V1/M5), which means patient’s movement was improved. Unfortunately, due to economic problem, he didn’t perform EEG and EMG. After 3 wk, he was transferred to local hospital.
This case exhibits a rare imaging manifestation of anti-Casper 2 encephalitis which was initially well-matched with PRES and associated vasospasm. Generally, PRES is predicted to be both clinically and radiologically reversible and especially has a good prognosis. One of the major causes of PRES is acute hypertension. Patients with normal BP who accompanied with systemic autoimmune disorders can also produce features of classic PRES radiologically. Tetsuka and Ogawa[1], proposed a case of PRES patient with anti-LGI 1 antibody whose MRI showed apparent vasospasm edema. It is presumed that factors such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), that lead to PRES can activate the immune system and release other cytokines. These cytokines produce expression of adhesion molecules (vascular cell adhesion molecule 1 and intercellular adhesion molecule 1), which cooperate with leukocytes and lead them to produce reactive oxygen species (ROS) and proteases that result in endothelial damage and consequent fluid leakage. TNF-α and IL-1 can furthermore stimulate astrocytes to secret vascular endothelial growth factor (VEGF), which deteriorates the form of junctions of the brain vasculature. These cascades result in vasogenic edema. In conclusion, endothelial hypotheses may be considered the most relevant in PRES patients with autoimmune disorders[2]. Meanwhile, PRES might cause the breakdown of blood-brain barrier (BBB) and the dis-organization of brain tissue[3]. In this case, it can be either presumed that BBB breakdown could uncover neuronal membrane antigen epitopes, such as Caspr 2, and further induce a process of autoimmune inflammatory encephalitis. More experiments should be taken in vitro and in vivo to further test the pathogenesis associated PRES-AE. Clinical diagnosis should also be made cautiously when a patient original has PRES neuroradiological features. PRES has been reported in patients with acute demyelinating encephalomyelitis (ADEM), multiple sclerosis (MS), other systemic autoimmune encephalitis (e.g. Hashimoto's disease, systemic lupuserythematosus, Behcet's disease), and paraneoplastic encephalitis[4].
In the present case, the patient was diagnosed as Caspr 2 AE rather than PRES due to the effectiveness of immunotherapy. In conclusion, AE can mimic PRES radiologically. AE should be further considered when the etiology, clinical manifestations and course of PRES are atypical. Persistent encephalopathic symptoms, imaging abnormalities in the multiple cortical and subcortical areas, and specifically, autoantibody analysis can be the evidences of AE. At last, immunotherapy and relevant systemic supportive treatment such as antiepileptic treatment, can lead to a better prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Beran RG, Australia; Jensen-Kondering U, Germany; Teragawa H, Japan S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: A review with emphasis on neuroimaging characteristics. J Neurol Sci. 2019;404:72-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses. 82:619-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Kim J, Lee ST, Park S, Joo EY, Chung CS, Lee MJ. Posterior reversible encephalopathy syndrome as initial manifestation of autoimmune encephalitis. Neurol Clin Pract. 2019;9:e42-e44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Wang Q, Huang B, Shen G, Zeng Y, Chen Z, Lu C, Lerner A, Gao B. Blood-Brain Barrier Disruption as a Potential Target for Therapy in Posterior Reversible Encephalopathy Syndrome: Evidence From Multimodal MRI in Rats. Front Neurol. 2019;10:1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |