Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11031
Peer-review started: April 24, 2022
First decision: June 8, 2022
Revised: July 4, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: October 26, 2022
Cri du chat syndrome (CdCS), also known as 5p deletion syndrome (5p-) is a syndrome caused by partial deletion of the 5p chromosome in human beings. The incidence accounts for 1/50000 and the cause of CdCS is related to partial deletion of chromosome 5 short arm (p). CdCS is a sporadic event. Only one case of CdCS was detected by chromosome screening in 125 and 170 pregnant Iranian women[1]. The most prominent clinical manifestations of CdCS are typical high-pitched cat calls, severe mental retardation or mental retardation and is most harmful to both language and growth retardation[2]. CdCS is a chromosome mutation disease which occurs during embryonic development and the symptoms of some cases are extremely atypical. It is difficult to make an early diagnosis and screening in clinic. We can suspect the disease from its atypical manifestations in the weak crying of cats, and chromosome karyotype analysis can find some questionable gene deletion fragments to assist the clinical diagnosis and prognosis of CdCS.
A 2-d-old male child who was admitted to our hospital with a poor postnatal reaction and poor milk intake. The baby's crying and sucking is weak, reaction and feeding time is poor and the baby has nausea and vomiting. Karyotype analysis showed that the chromosomes were 46, XY, deletion (5) p15. Whole genome microarray analysis (named ISCN2013) showed that the chromosomes of the child were male karyotypes and contained three chromosomal abnormalities. Among them, loss of 5p15.2pter (113576-13464559) was associated with cat call syndrome. After 3 mo of follow-up, the child still vomited repeatedly, had poor milk intake, did not return to normal growth, had developmental retardation and a poor directional response.
Therefore, when cat crying and laryngeal sounds occur in the neonatal period, it should be considered that they are related to CdCS. Chromosome karyotype and genome analysis are helpful for the diagnosis of CdCS.
Core Tip: A 2-d-old male child presented to our hospital with poor postnatal reaction and poor milk intake. The baby's crying and sucking is weak, reaction and feeding time is poor and the baby has nausea and vomiting. Karyotype analysis showed that the chromosomes were 46, XY, deletion (5) p15. Whole genome microarray analysis (named ISCN2013) showed that the chromosomes of the child were male karyotypes and contained three chromosomal abnormalities. Among them, loss of 5p15.2pter (113576-13464559) was associated with cat call syndrome. After a 3 mo of follow-up, the child still vomited repeatedly, had poor milk intake, did not return to normal growth, had developmental retardation and had a poor directional response.
- Citation: Bai MM, Li W, Meng L, Sang YF, Cui YJ, Feng HY, Zong ZT, Zhang HB. Neonatal Cri du chat syndrome with atypical facial appearance: A case report. World J Clin Cases 2022; 10(30): 11031-11036
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11031.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11031
In 1963, French physician and geneticist Jerome Lejeune first described Cri du chat syndrome (CdCS) as a partial chromosome deletion syndrome with an incidence of about 1/15000-1/50000. Among the population with IQ (Intelligence Quotient) values less than 50, the proportion could reach 1:350[3]. CdCS is caused by a partial or total deletion of the short arm of chromosome 5. The size of CdCS is about 10-45 Mb and only about 12% of CdCS deletion is caused by an unbalanced translocation or recombination of the chromosome of one parent. The main clinical manifestations of this syndrome are as follows: Crying like a cat in infancy, severe mental retardation and retardation of development, microcephaly, round face, wide eye spacing, hypotropia of cleft eye, low ear position, epicanthus, penetrating hand, etc. We report a case of neonatal CdCS, who lacked typical round face features and only had a weak cry like a cat. Finally, CdCS was diagnosed by chromosome karyotype detection and genomic analysis.
Poor postnatal reaction and poor milk intake.
The intrauterine pregnancy 39 + 1w, G1P1A0, spontaneous delivery. Birth weight was 2690 g (between 3 and 10 percentiles, Fenton curve), head circumference of 31.5 cm (below 3 percentiles), length of 48 cm. After birth, the baby's cry is weak, reaction is poor with poor feeding and weak sucking with nausea and vomiting.
Early pregnancy has a history of fetal protection and mild anemia. Prenatal premature rupture of membranes which lasted about 11 h.
Normal healthy parents of the child. Non-inmate marriage, deny any family genetic history, no special maternal contact history.
Body temperature 37.0 ℃, respiratory rate 68 bpm, blood pressure 70/52 mmHg, blood oxygen saturation 92%, weight 2520 g, head circumference 31.5 cm, body length 48 cm, poor stimulation response. The cry was weak, hoarse and catlike. The skin and sclera were yellow stained. The front fontanel was flat and the tension was not high and there was no facial deformity. Respiratory shortness of breath, inspiratory laryngeal ringing, heart rate 118 bpm, no murmur was heard in the valves on auscultation. Muscles tension have a slightly lower, weak sucking reflex, weak foraging reflex, normal grip reflex and incomplete hug reflex.
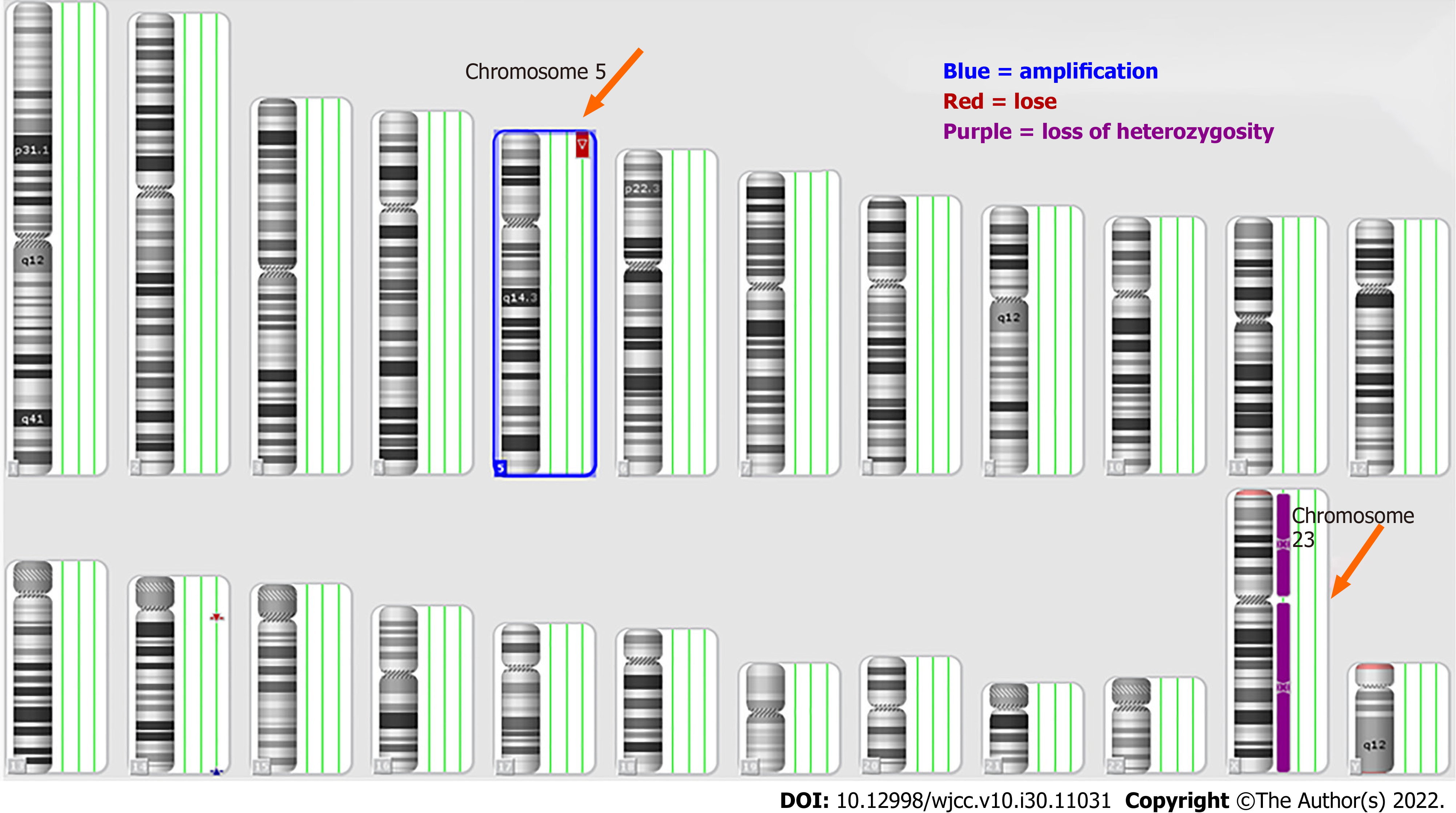
White blood cell count: 6.4 × 109/L; Red blood cell count: 5.33 × 1012/L; Hemoglobin: 190 g/L; Platelet count: 159 × 109/L; NE: 40.5% and Lymphocyte level: 49.9%; Procalcitonin level: 0.78 ng/mL. Karyotype analysis showed that the chromosomes were 46, XY, del (5) p15[20] (Figure 1). Whole genome microarray analysis (named ISCN2013) showed that the chromosomes of the child were male karyotypes and contained three chromosomal abnormalities. Among them, loss 5p15.2pter (113576-13464559) was associated with cat call syndrome, while gain (14q32.33) and loss (14q11.2) were benign cytomegalovirus changes and no pathological reports were found (Table 1 and Figure 2).
| Chromosome | Exception type | Chromosome abnormal zones and genome coordinates (ISCN2013) | Abnormal size in kbp | Clinical significance |
| 5 | Loss | arr (hg19) 5p 15.2 pter (113576-13464559) × 1 | 13351 | Abnormal correlation of hereditary diseases |
| 14 | Loss | arr (hg19)14q 11.2 (22510337-22969566) × 1 | 459 | Benign CNV changes |
| 14 | Gain | arr (hg19) 14q 32.33 (106251069-106751178) × 3 | 500 | - |
Craniocerebral ultrasound showed an intraventricular hemorrhage absorption period, cardiac ultrasound showed foramen ovale and patent ductus arteriosus, other examination results were normal.
Cri du chat syndrome.
The child was given symptomatic treatment and discharged after their clinical symptoms improved.
After 3 mo of follow-up, the child was still vomiting repeatedly, had poor milk intake, 38 cm head circumference, 5000 g body weight, 55 cm body length (all below 3%), did not return to normal growth and development retardation, and had poor directional response. After stimulation, the crying of the child still resembled high-profile cat calls, with no special facial changes. After 12 mo of follow-up, the growth and development of the child were normal, but they could not make laughter or sound. They could only blur out a single syllable and react slowly to external stimuli.
Lejeune first described CdCS as the first chromosome partial deletion syndrome in 1963. The incidence of CdCS was 1/50000[3]. The cause was related to the partial deletion of the short arm (p) of chromosome 5. Typical clinical symptoms of CdCS are high-profile cat crying in infancy, growth retardation, small head with a round face deformity and mental retardation[4]. High-pitched cat calls in infancy are a typical clinical phenotype of the disease, which may be related to laryngeal development. Hassink G found that MARCH6 (TEB4) (9035025-9546,120) is an E3 ubiquitin ligase located in the endoplasmic reticulum and a key gene associated with high-profile cat calls in children with 5p deletion. MARCH6 (TEB4) is involved in the protein degradation pathway. In gene expression experiments of animal embryos, it was found that MARCH6 is highly expressed in the chest and scalp tissues. It is speculated that MARCH6 (TEB4) may be involved in the cat barking sound[5]. 5p ranges from 5791886 to 7539901 in a 1.7 Mb area, 10361807 to 15728105 in a 5.4 Mb area, 22178 to 5539182 in a 5.5 Mb area and are all related to high-pitched cat calls[6]. This case was diagnosed by a genetic test because of the suspected high-profile cat calls. A notable clinical phenotype of CdCS is developmental retardation, but lack of clinical specificity[7]. In infants, poor feeding, frequent gastroesophageal reflux, and suffocation can also occur. This affects both growth and development of the child. The clinical phenotype associated with CDCS is hTERT (1253166-1295625)[8]. The infant's birth weight was 2690 g and head circumference was 31.5 cm which was below the 10th percentile. There was intrauterine growth retardation, slow feeding, repeated breast-feeding, growth retardation, postnatal weight index of 2.43, body length/head circumference was 1.52 and other indicators are lower than those of normal newborns. These manifestations are consistent with the clinical manifestations caused by the deletion of this gene’s phenotype.
Small head and round face deformity in infancy CdCS is more obvious which is manifested by a small head and round face deformity, widened eye cracks and low nasal bridge equality. This facial feature gradually becomes longer and narrower as the age increases to adolescence and adulthood and the facial features may become less obvious[3]. This case lacks the typical facial features but exhibits cat-like crying, inspiratory laryngitis and slow action which are difficult to diagnose. The serious harm of this disease is that it causes severe mental retardation and language development disorders which can assist early recognition of CdCS[9]. The most common deletion of the key genes of SEMA5A (9035025-9546120) and CTNND2 (10971951-11904154) in children with CdCS is related to the development of the nervous system. The deletion of these genes exists in the 5p15.2 region which will affect brain development and lead to neurodevelopmental retardation[10,11]. We consulted the OMIM website and identified TERT (1253166-1295625)[12], SEMA5A (9035025-9546120)[13], MARCH6 (10353638-10440387)[14], CTNND2 (10971951-11904154)[15], which is a single dose sensitive gene. The prognosis of CdCS is unsatisfactory, mainly due to language and mental retardation. During the follow-up of this case, mental retardation, language disorders and nervous system development retardation were found in the same age infants.
Therefore, the diagnosis of neonatal CdCS should be considered when cat crying and laryngeal sounds occur in the neonatal period. Chromosome analysis and gene screening can identify CdCS early. Its clinical phenotype and prognosis are related to the difference of deleted CdCS gene fragments.
The authors are grateful to the family members and patients for their participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farouk S, Egypt; Gazouli M, Greece S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Niebuhr E. The Cri du Chat syndrome: epidemiology, cytogenetics, and clinical features. Hum Genet. 1978;44:227-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 195] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Seyyed Kavoosi E, Younessi S, Farhud DD. Screening of Fetal Chromosome Aneuploidies in the First and Second Trimester of 125,170 Iranian Pregnant Women. Iran J Public Health. 2015;44:791-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Kodra Y, Cavazza M, de Santis M, Guala A, Liverani ME, Armeni P, Masini M, Taruscio D. Social Economic Costs, Health-Related Quality of Life and Disability in Patients with Cri Du Chat Syndrome. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Rodríguez-Caballero A, Torres-Lagares D, Rodríguez-Pérez A, Serrera-Figallo MA, Hernández-Guisado JM, Machuca-Portillo G. Cri du chat syndrome: a critical review. Med Oral Patol Oral Cir Bucal. 2010;15:e473-e478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hassink G, Kikkert M, van Voorden S, Lee SJ, Spaapen R, van Laar T, Coleman CS, Bartee E, Früh K, Chau V, Wiertz E. TEB4 is a C4HC3 RING finger-containing ubiquitin ligase of the endoplasmic reticulum. Biochem J. 2005;388:647-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Nguyen JM, Qualmann KJ, Okashah R, Reilly A, Alexeyev MF, Campbell DJ. 5p deletions: Current knowledge and future directions. Am J Med Genet C Semin Med Genet. 2015;169:224-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Borrell A, Grande M, Pauta M, Rodriguez-Revenga L, Figueras F. Chromosomal Microarray Analysis in Fetuses with Growth Restriction and Normal Karyotype: A Systematic Review and Meta-Analysis. Fetal Diagn Ther. 2018;44:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Wu Q, Niebuhr E, Yang H, Hansen L. Determination of the 'critical region' for cat-like cry of Cri-du-chat syndrome and analysis of candidate genes by quantitative PCR. Eur J Hum Genet. 2005;13:475-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Honjo RS, Mello CB, Pimenta LSE, Nuñes-Vaca EC, Benedetto LM, Khoury RBF, Befi-Lopes DM, Kim CA. Cri du Chat syndrome: Characteristics of 73 Brazilian patients. J Intellect Disabil Res. 2018;62:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Lin S, Rawlins L, Turner C, Doyle E, Sleep T. Novel ocular findings with 5p deletion and partial trisomy of distal 4q. Can J Ophthalmol. 2018;53:e89-e90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hofmeister W, Nilsson D, Topa A, Anderlid BM, Darki F, Matsson H, Tapia Páez I, Klingberg T, Samuelsson L, Wirta V, Vezzi F, Kere J, Nordenskjöld M, Syk Lundberg E, Lindstrand A. CTNND2-a candidate gene for reading problems and mild intellectual disability. J Med Genet. 2015;52:111-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Lin S, Nascimento EM, Gajera CR, Chen L, Neuhöfer P, Garbuzov A, Wang S, Artandi SE. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | Weiss LA, Arking DE; Gene Discovery Project of Johns Hopkins & the Autism Consortium, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 455] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Park SE, Kim JM, Seok OH, Cho H, Wadas B, Kim SY, Varshavsky A, Hwang CS. Control of mammalian G protein signaling by N-terminal acetylation and the N-end rule pathway. Science. 2015;347:1249-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Matter C, Pribadi M, Liu X, Trachtenberg JT. Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo. Neuron. 2009;64:320-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |