Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.10906
Peer-review started: March 19, 2022
First decision: May 1, 2022
Revised: May 8, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: October 26, 2022
The prognosis of patients with appendiceal neuroendocrine tumors (ANETs) is related to lymph node (LN) metastasis and other factors. However, it is unclear how the number of examined LNs (ELNs) impact on survival.
To determine the factors affecting the cancer-specific survival (CSS) of patients with ANET and to evaluate the impact of the number of ELNs on survival.
A total of 4583 ANET patients were analyzed in the Surveillance, Epidemiology, and End Results database. Univariate survival analysis was used to identify factors related to survival and the optimal number of ELNs and lymph node ratio (LNR) were determined by the Kaplan–Meier method. The survival difference was determined by CSS.
Except for sex, the other factors, such as age, year, race, grade, histological type, stage, tumor size, ELNs, LNR, and surgery type, were associated with prognosis. The 3-, 5-, and 10-year CSS rates of ANET patients were 91.2%, 87.5, and 81.7%, respectively (median follow-up period of 31 mo and range of 0-499 mo). There was no survival difference between the two surgery types, namely, local resection and colectomy or greater, in both stratifications of tumor size ≥ 2 cm (P = 0.523) and < 2 cm (P = 0.068). In contrast to patients with a tumor size < 2 cm, those with a tumor size ≥ 2 cm were more likely to have LN metastasis (χ2 = 378.16, P < 0.001). The optimal number of ELNs was more than 11, 7, and 18 for all patients, node-negative patients, and node-positive patients, respectively. CSS rates of patients with a larger number of ELNs were significantly improved (≤ 10 vs ≥ 11, χ2 = 20.303, P < 0.001; ≤ 6 vs ≥ 7, χ2 = 11.569, P < 0.001; ≤ 17 vs ≥ 18, χ2 = 21.990, P < 0.001; respectively). ANET patients with an LNR value ≤ 0.16 were more likely to have better survival than those with values of 0.17-0.48 (χ2 = 48.243, P < 0.001) and 0.49-1 (χ2 = 168.485, P < 0.001).
ANET ≥ 2 cm are more likely to develop LN metastasis. At least 11 ELNs are required to better evaluate the prognosis. For patients with positive LN metastasis, 18 or more LNs need to be detected and lower LNR values (LNR ≤ 0.16) indicate a better survival prognosis.
Core Tip: This study aimed to explore factors that have an influence on survival of patients with appendiceal neuroendocrine tumors. We identified the optimal number of examined lymph nodes that could achieve the best survival for patients with appendiceal neuroendocrine tumors with different lymph node statuses. Furthermore, lymph node ratio takes both examined lymph nodes and positive lymph nodes into account. We also identified the optimal value of lymph node ratio that could achieve the best survival for node-positive patients.
- Citation: Du R, Xiao JW. Prognostic impact of number of examined lymph nodes on survival of patients with appendiceal neuroendocrine tumors. World J Clin Cases 2022; 10(30): 10906-10920
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/10906.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.10906
Carcinoid tumors were first described by some researchers[1] in 1907, and neuroendocrine tumors (NETs) were first described by some researchers[2] in 1888. NETs, historically known as carcinoid tumors, are mainly found in the gastrointestinal tract, but they can occur in multiple sites throughout the body[3]. Gastrointestinal NETs are most common in the stomach, small intestine, and pancreas, and their incidence has been reported to be steadily increasing in recent years[4]. The Surveillance Epidemiology and End Results (SEER) database estimates 3.56 cases of gastrointestinal NETs per 100000 individuals each year[5]. Appendiceal neuroendocrine tumors (ANETs), belonging to appendiceal carcinoids, are considered a subtype of midgut NET[6], which account for almost 60% of all appendiceal tumors[7,8]. Most ANETs are found via pathological examination after appendectomy. According to a retrospective study, 29 (0.2%) of 13863 appendectomy specimens in 10 years were histopathologically confirmed to have NETs[7]. Another study revealed that 17 (0.27%) of the 6369 patients who underwent appendectomy had ANETs[9].
For prognosis, a previous study has shown that the 5-year overall survival (OS) of all gastrointestinal NETs is 67.2% in a cohort of 73782 patients[10]. Another study has shown that the median survival duration is 41 mo for patients with gastrointestinal NETs, and 5- and 10-year OS rates are 39.4% and 18.1%, respectively[11]. In comparison, ANETs had a better prognosis than gastrointestinal NETs[12]. The 10-year OS has been reported to be as high as 95% (53 of 56)[13]. The survival of ANET patients is primarily determined by tumor grade and stage[14]. In 2001, an analysis of 619 cases with ANETs using Cox multivariate regression showed that age, stage, sex, and primary appendix localization are independent predictors of survival[15]. A retrospective study has shown that the lymph node (LN) status of ANET patients is related to survival[16]. However, it remains unclear whether the number of LNs detected and the positive rate are related to the prognosis.
So far, there has not detailed survival rate of patients with ANET, especially the survival rates related to different disease stages. Further, there are clinical cases diagnosed with ANETs preoperatively. The issue is what type of surgery should be chosen and how many LNs should be resected for optimal survival in this situation. The purpose of the present study was to determine the related factors that affect the cancer-specific survival (CSS) of ANET patients and the impact of the number and positive rate of LNs detected on survival and prognosis. This study also investigated whether the survival prognosis is related to tumor size, scope of resection, and other factors.
Data were collected from the SEER database. A total of 14920 cases of appendectomy were extracted by anatomical site, and 5808 cases of NETs or carcinoid tumors were identified according to the 3rd edition of the International Classification of Diseases for Oncology. A total of 1002 cases of nonprimary and nonfirst primary appendiceal tumors were excluded. Ultimately, 4583 cases with ANETs were included.
The following variables were reviewed: Age (age at diagnosis), year (year at diagnosis), race, sex, grade (well differentiated, moderately differentiated, poorly differentiated, and undifferentiated), histological type (9 categories), tumor size (reclassified into ≤ 2 cm and > 2 cm), and stage (patients were restaged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. “T stage” included Tx, Tis, T1 (T1a and T1b), T2, T3, and T4 (T4a, T4b, and T4). The data variables (N0, N1, N2, and Nx) of the N status were reclassified into N0 and N1. The M status in the database was transformed into the standard “M stage” by the 7th edition of AJCC, and the M0 and M1 (M1a, M1b, and M1) data variables were reclassified into M0 and M1 categories. The stage status of the disease was modified to stages I-IV. Surgery types were reclassified into local resection and colectomy or greater. Examined lymph nodes (ELN) is the exact number of LNs detected. Lymph node ratio (LNR) is the lymph node positive rate, which was calculated as the number of positive LNs divided by the number of ELNs. Survival duration was defined as the interval from the date of diagnosis to the date of death. CSS was the primary vital status (death attributable to the cancer) in this study.
Data were entered into Excel datasheets from the SEER database and then analyzed with SPSS 18.0 (IBM, Armonk, NY, United States) statistics software for Windows. Figures were created using GraphPad Prism software version 7.00 (San Diego, CA, United States). Continuous variables are expressed as the mean ± SD. Categorical data are expressed as absolute values or fractions. The Cox proportional hazards model was applied to assess the prognostic factors associated with survival, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The CSS survival curves were plotted using the Kaplan-Meier method and compared by the log-rank test. P < 0.05 was considered statistically significant. Continuous variables were also converted into categorical variables. X-tile software version 3.6.1 (Yale University, New Haven, CT, United States) was used to determine the optimal cutoff points of ELNs and LNR[17].
As shown in Table 1, 4583 patients were included from 1975 to 2016, of which 57% were female, with a mean age of 44.59 years. White people were the majority race. There were four histopathological grades according to the degrees of differentiation, and 72.6% of cases were well differentiated. The mean tumor size was 17.56 mm. Most patients were at an earlier stage in terms of T, N, and M stages, and 57.36% were at stage I. On average, 16.5 LNs were examined, and the mean LNR was 0.26. The mean interval from diagnosis to the resection date was 64.57 mo.
| Factor | Category | mean ± SD/n (%) |
| Age | mean ± SD, yr | 44.59 ± 18.15 |
| Year | 1975-1980 | 141 (3.08) |
| 1981-1990 | 128 (2.79) | |
| 1991-2000 | 274 (5.98) | |
| 2001-2010 | 1163 (25.38) | |
| 2011-2016 | 2877 (62.77) | |
| Race | White | 3932 (87.34) |
| Black | 375 (8.33) | |
| Other | 195 (4.33) | |
| Sex | Male | 1972 (43) |
| Female | 2611 (57) | |
| Grade | Grade 1 | 1857 (72.59) |
| Grade 2 | 408 (15.96) | |
| Grade 3 | 248 (9.69) | |
| Grade 4 | 45 (1.76) | |
| Histological type | Large cell neuroendocrine carcinoma | 6 (0.13) |
| Small cell carcinoma | 1 (0.02) | |
| Carcinoid tumor | 2266 (49.44) | |
| Enterochromaffin cell carcinoid | 25 (0.55) | |
| Goblet cell carcinoid | 1033 (22.54) | |
| Mixed adenoneuroendocrine carcinoma | 366 (7.99) | |
| Adenocarcinoid tumor | 417 (9.10) | |
| Neuroendocrine carcinoma | 419 (9.14) | |
| Atypical carcinoid tumor | 50 (1.09) | |
| Tumor size | mean ± SD, mm | 17.56 ± 19.69 |
| Stage | I | 1220 (57.36) |
| II | 504 (23.70) | |
| III | 270 (12.69) | |
| IV | 133 (6.25) | |
| T stage | Tx | 86 (3.86) |
| Tis | 7 (0.31) | |
| T1 | 1290 (57.87) | |
| T2 | 194 (8.70) | |
| T3 | 446 (20.01) | |
| T4 | 204 (9.15) | |
| M stage | M0 | 2094 (94.07) |
| M1 | 132 (5.93) | |
| N stage | N0 | 2551 (81.76) |
| N1 | 569 (18.24) | |
| Surgery | Local resection | 405 (10.34) |
| Colectomy or greater | 3513 (89.66) | |
| ELNs | mean ± SD | 16.54 ± 10.81 |
| LNR | mean ± SD | 0.26 ± 0.28 |
| Survival duration | mean ± SD, mo | 64.57 ± 89.96 |
The continuous variables were transformed into classified variables. In particular, age, LNR, and number of ELNs were divided into subsections by the cutoff values determined with X-tile software[17]. Age was divided into three levels as follows: ≤ 40 years old, 41-65 years old, and ≥ 66 years old. Patients were divided into two groups according to the ELN cutoff points. All node-positive patients were divided into three levels according to LNR cutoff points as: 0 < LNR ≤ 0.16, 0.17 ≤ LNR ≤ 0.48, and 0.49 ≤ LNR ≤ 1. Histological types in few patients were ignored. For the stage and grade, we clustered them into a dichotomy as: Grade 1/2 and grade 3/4; and stage I/II and stage III/IV. CSS was significantly different between the groups for each variable by the log-rank test.
In the univariate analysis, age ≥ 66 years (HR = 16.14, 95%CI: 11.08-23.52, P < 0.001; reference: ≤ 40 years), diagnosis in 1991-2000 (HR = 4.72, 95%CI: 2.51-8.85, P < 0.001; reference: 1975-1980), Black people (HR = 1.58, 95%CI: 1.20-2.08, P = 0.02; reference: White people), female gender (HR = 1.02, 95%CI: 0.85-1.23, P = 0.80; reference: Male), grade 3/4 (HR = 19.14, 95%CI: 13.63-26.87, P < 0.001; reference: Grade 1/2), large cell neuroendocrine carcinoma (HR = 14.45, 95%CI: 10.30-20.27, P < 0.001; reference: Carcinoid tumor), tumor size > 2 cm (HR = 8.54, 95%CI: 5.99-12.17, P < 0.001; reference: ≤ 2 cm), stage III/IV (HR = 17.12, 95%CI: 11.78-24.87, P < 0.001; reference: Stage I/II), number of ELN ≤ 10 (HR = 1.75, 95%CI: 1.37-1.23, P < 0.001; reference: ≥ 11), LNR = 0.49-1 (HR = 7.70, 95%CI: 5.38-11.01, P < 0.001; reference: 0-0.16), and surgery of colectomy or greater (HR = 3.47, 95%CI: 1.95-6.17, P < 0.001; reference: Local resection) were predictors of poor CSS. The results are shown in Table 2.
| Factor | Category | HR | 95%CI | Log-rank P value |
| Age | ≤ 40 yr | - | - | P < 0.001 |
| 41-65 yr | 7.25 | 5.09-10.32 | ||
| ≥ 66 yr | 16.14 | 11.08-23.52 | ||
| Year | 1975-1980 | - | - | P < 0.001 |
| 1981-1990 | 3.06 | 1.56-6.01 | ||
| 1991-2000 | 4.72 | 2.51-8.85 | ||
| 2001-2010 | 3.83 | 2.09-7.02 | ||
| 2011-2016 | 1.84 | 0.98-3.43 | ||
| Race | White | - | - | P = 0.02 |
| Other | 1.15 | 0.73-1.83 | ||
| Black | 1.58 | 1.20-2.08 | ||
| Sex | Male | - | - | P = 0.80 |
| Female | 1.02 | 0.85-1.23 | ||
| Grade | Grade 1/2 | - | - | P < 0.001 |
| Grade 3/4 | 19.14 | 13.63-26.87 | ||
| Histological type | Carcinoid tumor | - | - | P < 0.001 |
| Neuroendocrine carcinoma | 2.36 | 1.44-3.88 | ||
| Goblet cell carcinoid | 4.98 | 3.61-6.87 | ||
| Adenocarcinoid tumor | 6.87 | 4.89-9.64 | ||
| Mixed adenoneuroendocrine carcinoma | 14.45 | 10.30-20.27 | ||
| Tumor size | ≤ 2 cm | - | - | P < 0.001 |
| > 2 cm | 8.54 | 5.99-12.17 | ||
| Stage | I/II | - | - | P < 0.001 |
| III/IV | 17.12 | 11.78-24.87 | ||
| T stage | T1 | - | - | P < 0.001 |
| T2 | 5.16 | 1.29-20.64 | ||
| T3 | 17.25 | 6.09-48.81 | ||
| T4 | 117.44 | 43.03-320.55 | ||
| M stage | M0 | - | - | P < 0.001 |
| M1 | 31.37 | 22.16-44.39 | ||
| N stage | N0 | - | - | P < 0.001 |
| N1 | 8.44 | 6.69-10.65 | ||
| Surgery | Local resection | - | - | P < 0.001 |
| Colectomy or greater | 3.47 | 1.95-6.17 | ||
| ELNs | ≥ 11 | - | - | P < 0.001 |
| ≤ 10 | 1.75 | 1.37-2.23 | ||
| LNR | 0-0.16 | - | - | P < 0.001 |
| 0.17-0.48 | 3.23 | 2.25-4.64 | ||
| 0.49-1 | 7.70 | 5.38-11.01 |
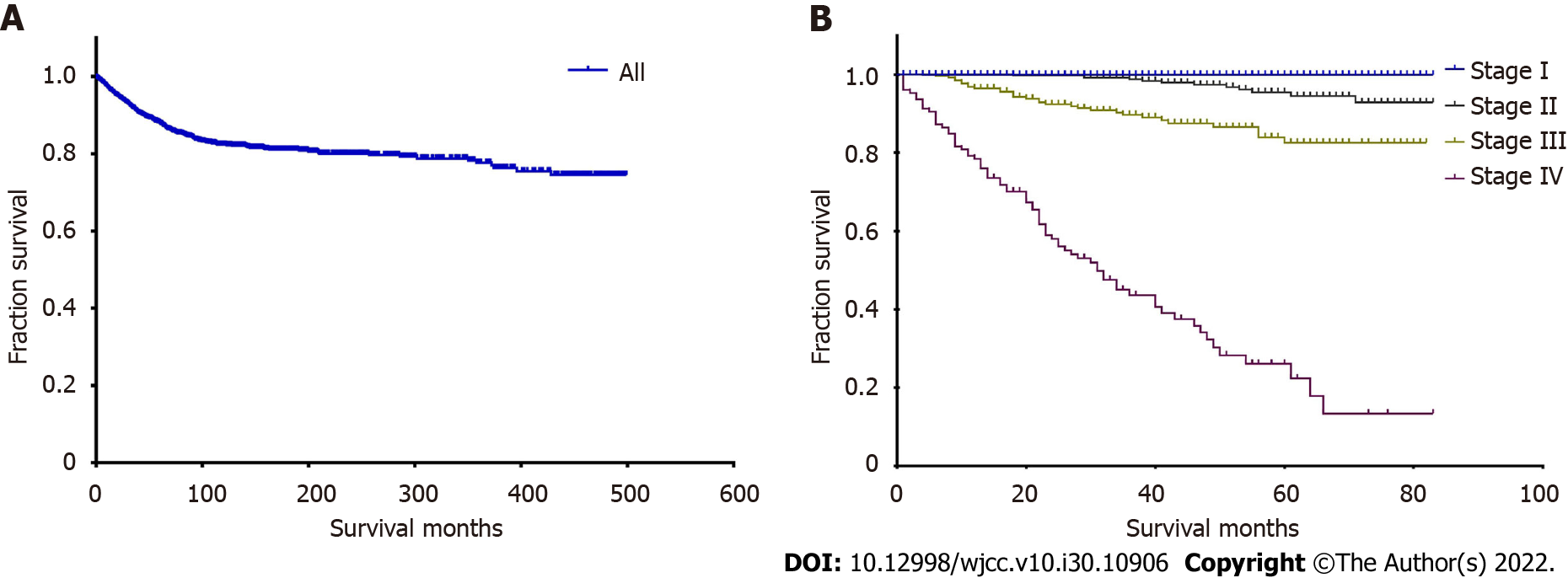
For the whole cohort, the median follow-up time was 31 mo (range, 0-499 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 91.2%, 87.5%, and 81.7%, respectively. We calculated the 3-, 5-, and 10-year CSS rates of patients at each stage, and the rate decreased as the stage increased as shown in Table 3. The 10-year CSS rates and most median CSS times were unknown. We also plotted the survival curve for all patients (Figure 1A) and curves based on the four stages (Figure 1B).
| Patients | 3-yr, % | 5-yr, % | 10-yr, % | Follow-up, mo | Median follow-up, mo | Median survival time, mo |
| All | 91.2 | 87.5 | 81.7 | 0-499 | 31 | - |
| Stage I | 99.7 | 99.7 | - | 0-83 | 23 | - |
| Stage II | 98.7 | 95.5 | - | 0-83 | 42 | - |
| Stage III | 89.0 | 82.0 | - | 0-82 | 38 | - |
| Stage IV | 42.0 | 25.1 | - | 0-83 | 23 | 30 |
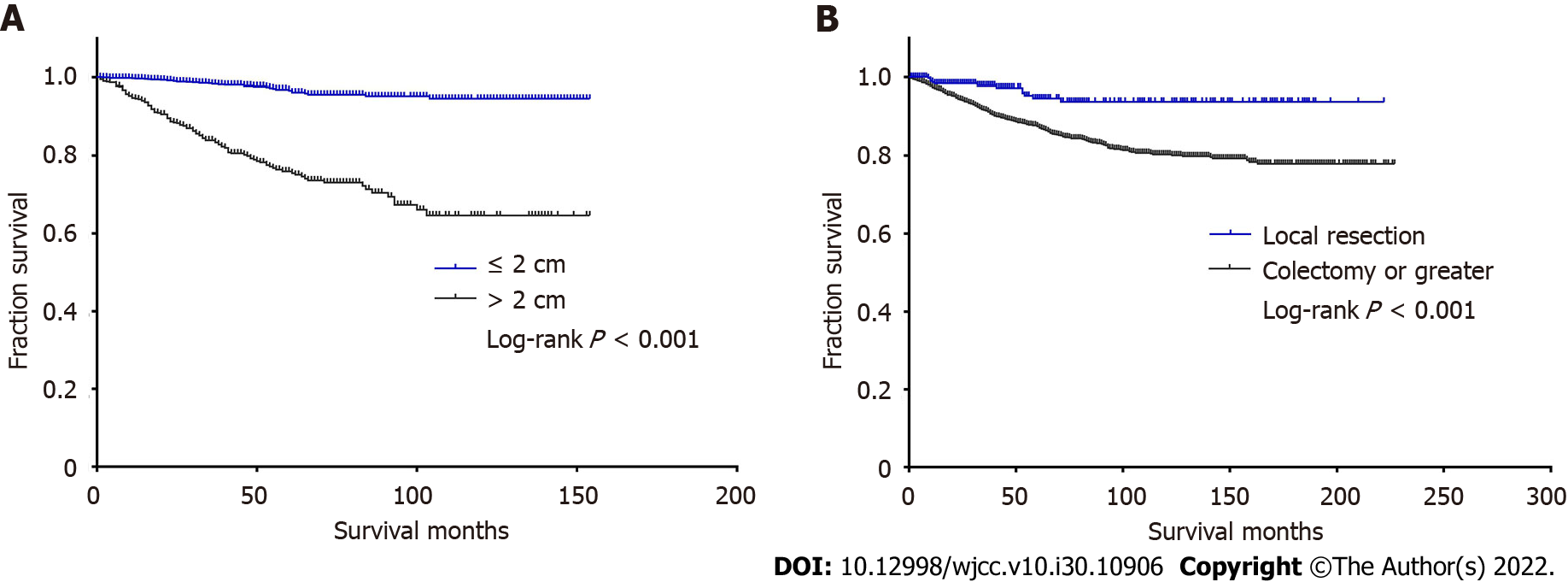
Tumor size > 2 cm is generally considered to be an important prognostic factor for patients with ANETs, and it may also affect the choice of surgery. According to the North American Neuroendocrine Tumor Society (NANET) guidelines, > 2 cm is one of the criteria for right hemicolectomy (RHC) for ANET patients[18]. The European Neuroendocrine Tumor Society (ENETS) guidelines also recommend aggressive surgery for ANET patients with tumors > 2 cm due to the risk of recurrence and metastasis. In addition, tumor stratification is partly according to tumor size[19]. In the present study, we divided the tumor size and surgery into two categories. Univariate analysis suggested that there was a significant survival difference between tumor sizes and different surgeries by the log-rank test (P < 0.001). The survival curves are shown in Figure 2. Patients with tumors ≤ 2 cm and who underwent local resection had better survival compared to the other categories.
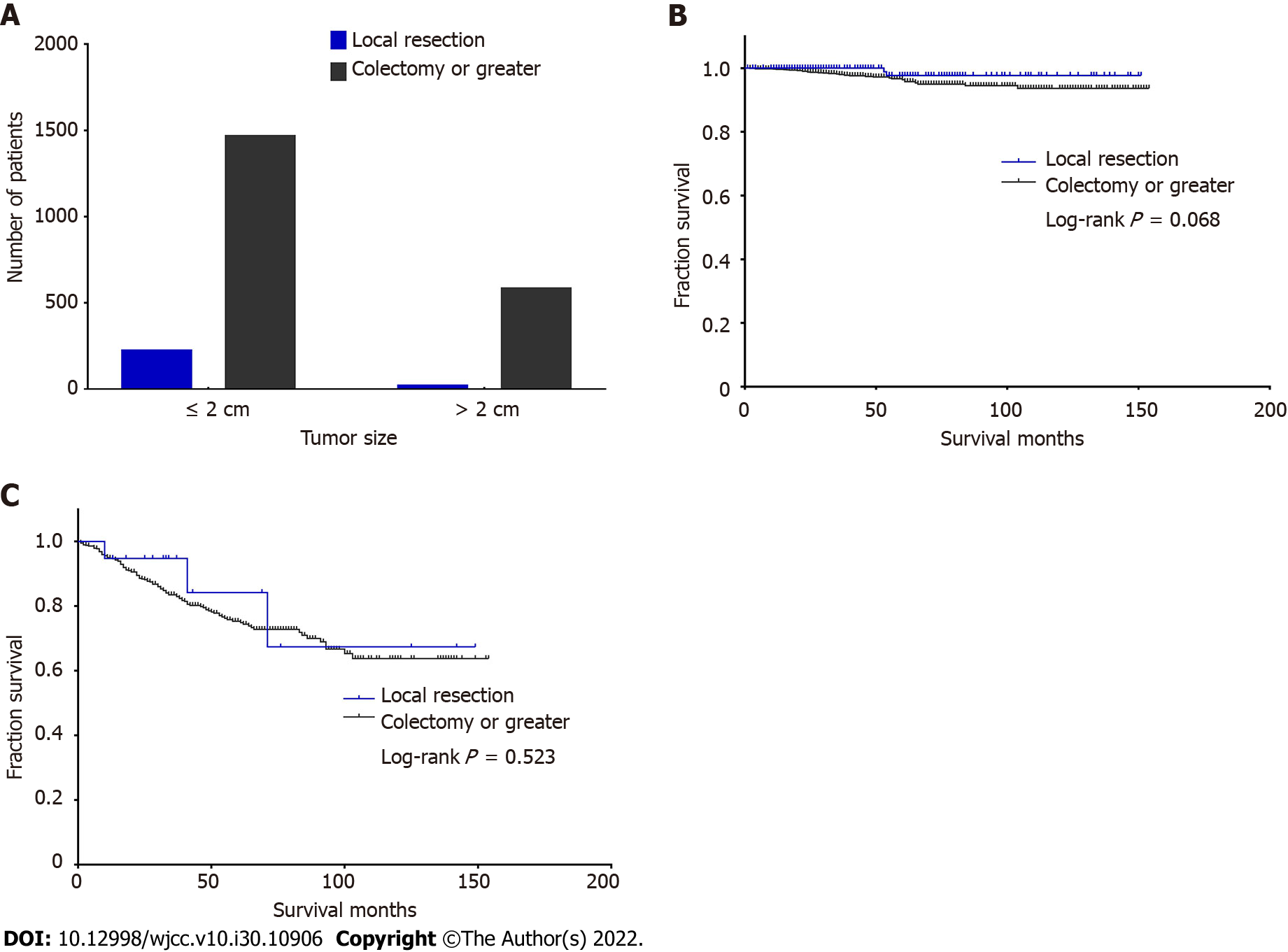
To determine whether survival differences exist between surgical methods in patients with different tumor sizes, we also conducted a survival analysis of two surgeries but divided the patients into two stratifications by tumor size. There were 225 patients undergoing local resection and 1468 patients undergoing colectomy or greater with tumor size ≤ 2 cm, while there were 21 patients undergoing local resection and 584 patients undergoing colectomy or greater with rumor size > 2 cm (Figure 3A). The log-rank test showed that there was no significant difference in both tumor size between the two surgeries (P = 0.068, Figure 3B; P = 0.523, Figure 3C). The data analysis showed that when the tumor size was less than 2 cm, there was no survival benefit due to expansion surgery (Figure 3B). Therefore, for ANETs less than 2 cm, right hemicolectomy should be carefully selected. According to our analysis results, when tumors were larger than 2 cm, the two different surgical methods did not show the expected survival difference (Figure 3C), but only 21 patients with tumors larger than 2 cm chose local resection, which may have produced statistical bias.
Small ANETs are generally considered to be benign, and LN metastasis is rarely reported for tumors smaller than 2 cm[18]. There is a clearly increased risk of LN metastasis for ANETs > 2 cm[19], and the risk is up to 40%[20]. In addition, a tumor diameter of 2 cm has been suggested to be associated with LN metastasis. To confirm this in our cohort, 2202 patients were divided into two categories according to both tumor sizes and LN status (Table 4). There were a total of 1837 (85.1%) node-negative patients and 329 (14.9%) node-positive patients. For all 1613 patients with tumor size ≤ 2 cm, there were 1516 (94.0%) node-negative patients and only 97 (6.0%) node-positive patients. For all 589 patients with tumor size > 2 cm, there were 357 (60.6%) node-negative patients and 232 (39.4%) node-positive patients. The chi-squared test showed that there was a significant difference in LN metastasis between patients with different tumor sizes (χ2 = 378.16, P < 0.001). Patients with tumor size > 2 cm were more likely to be susceptible to LN metastasis (Figure 4).
| Tumor size | LN status | Total | |
| N0 | N1 | ||
| ≤ 2 cm | 1516 (94.0) | 97 (6.0) | 1613 (100) |
| > 2 cm | 357 (60.6) | 232 (39.4) | 589 (100) |
| Total | 1873 (85.1) | 329 (14.9) | 2202 (100) |
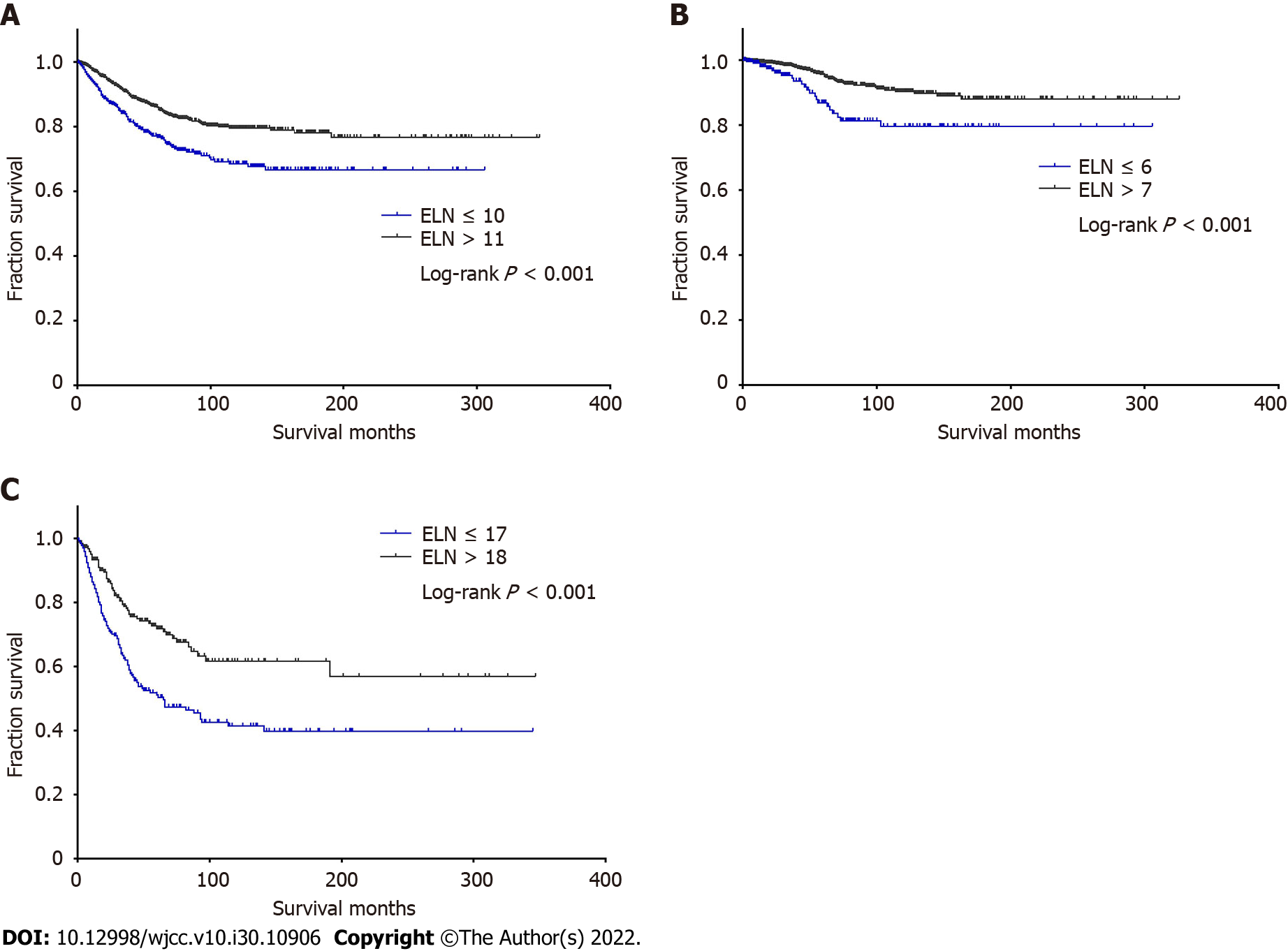
We used X-tile software to identify the optimal number of ELNs that generated the greatest survival difference. For the entire cohort, 11 LNs was the optimal number of ELNs that generated the greatest survival difference (χ2 = 20.303, P < 0.001). The cutoff point was 7 LNs (χ2 = 11.569, P = 0.001) for node-negative patients and 18 LNs (χ2 = 21.990, P < 0.001) for node-positive patients. We further calculated the 3-, 5-, and 10-year CSS rates for patients based on LN status and different numbers of ELNs (Table 5).
| Patients | ELNs | 3-yr, % | 5-yr, % | 10-yr, % | Follow-up, mo | Median follow-up, mo | Median survival, mo |
| All | ≤ 10 | 83.2 | 76.1 | 67.9 | 0-306 | 36 | - |
| ≥ 11 | 90.3 | 85.5 | 79.1 | 0-347 | 38 | - | |
| Node-negative | ≤ 6 | 94.9 | 86.5 | 79.3 | 0-306 | 28 | - |
| ≥ 7 | 98.1 | 95.4 | 90.1 | 0-326 | 43 | - | |
| Node-positive | ≤ 17 | 60.7 | 50.0 | 40.6 | 0-345 | 31 | 60 |
| ≥ 18 | 78.4 | 71.5 | 61.4 | 0-347 | 35 | - |
For two categories divided by the ELN cutoff point of all patients, the median follow-up of patients with ≤ 10 ELNs was 36 mo (range, 0-306 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 83.2%, 76.1%, and 67.9%, respectively. For patients with ≥ 11 ELNs, the median follow-up was 38 mo (range, 0-347 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 90.3%, 85.5%, and 79.1%, respectively. The Kaplan–Meier survival curve based on ELN cutoff points was plotted (Figure 5A). Among all patients, patients with ≥ 11 ELNs had a better CSS than patients with ELNs ≤ 10 (χ2 = 20.303, P < 0.001). The results suggested that the number of LNs detected should be greater than or equal to 11 for a better survival and prognosis.
Considering node-negative patients, patients with ELNs ≤ 6 had a median follow-up of 28 mo (range, 0-306 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 94.9%, 86.5%, and 79.3%, respectively. For patients with ELNs ≥ 7, the median follow-up was 43 mo (range, 0-326 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 98.1%, 95.4%, and 90.1%, respectively. We plotted survival curves based on ELN cutoff points of ≤ 6 and ≥ 7 for node-negative patients (Figure 5B). Patients with ELNs ≥ 7 had a better CSS (χ2 = 11.569, P < 0.001). The results suggested that the number of LNs detected in node-negative ANET patients is preferably greater than or equal to 7 for a better survival.
For the node-positive patients, patients with ELNs ≤ 17 had a median follow-up of 31 mo (range, 0-345 mo), and the median CSS time was 60 mo. The 3-, 5-, and 10-year CSS rates were 60.7%, 50.0%, and 40.6%, respectively. For patients with ELNs ≥ 18, the median follow-up was 35 mo (range, 0-347 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 78.4%, 71.5%, and 61.4%, respectively. Kaplan–Meier survival curves based on ELN cutoff points for node-positive patients were plotted (Figure 5C). Patients with ELNs ≥ 18 had a better CSS than patients with ELNs ≤ 17 (χ2 = 24.464, P < 0.001). The results suggested that the number of LNs detected in node-positive ANET patients is preferably greater than or equal to 18 for a better survival and prognosis.
We found that 0.16 was the optimal cutoff point of LNR that generated the greatest survival difference for node-positive patients. The log-rank test showed that there were survival differences among the three stratifications divided by two cutoff values of LNR (χ2 = 160.406, P < 0.001). We calculated the 3-, 5-, and 10-year CSS rates for all node-positive patients by different LNRs (Table 6). For all node-positive patients, the median follow-up was 33 mo (range, 0-347 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates for all node-positive patients were 67.3%, 58.4%, and 48.9%, respectively. For the three stratifications divided by the LNR cutoff points, the median follow-up of patients with an LNR ≤ 0.16 was 45 mo (range, 0-347 mo), and the median CSS time was unknown. The 3-, 5-, and 10-year CSS rates were 88.5%, 80.8%, and 68.9%, respectively. For patients with an LNR between 0.17 and 0.48, the median follow-up was 32 mo (range, 1-345 mo), and the median CSS time was 46 mo. The 3-, 5-, and 10-year CSS rates were 59.7%, 46.2%, and 37.4%, respectively. For patients with an LNR ≥ 0.49, the median follow-up was 16 mo (range, 0-203 mo), and the median CSS time was 18 mo. The 3-, 5- and 10-year CSS rates were 24.7%, 17.7% and 14.2%, respectively.
| LNR | 3-yr, % | 5-yr, % | 10-yr, % | Follow-up, mo | Median follow-up, mo | Median survival, mo |
| ALL | 67.3 | 58.4 | 48.9 | 0-347 | 33 | - |
| 0-0.16 | 88.5 | 80.8 | 68.9 | 0-347 | 45 | - |
| 0.17-0.48 | 59.7 | 46.2 | 37.4 | 1-345 | 32 | 46 |
| 0.49-1 | 24.7 | 17.7 | 14.2 | 0-203 | 16 | 18 |
LNR ≤ 0.16 was associated with a better CSS. Kaplan–Meier survival curves based on the LNR cutoff points were plotted (Figure 6). Survival differences existed between patients with an LNR ≤ 0.16 and those with an LNR between 0.17 and 0.48 (χ2 = 48.243, P < 0.001), between patients with an LNR between 0.17 and 0.48 and those with an LNR ≥ 0.49 (χ2 = 26.908, P < 0.001), as well as between patients with an LNR ≤ 0.16 and those with an LNR ≥ 0.49 (χ2 = 168.485, P < 0.001). Compared to patients with an LNR ≥ 0.17, patients with an LNR ≤ 0.16 were more likely to have a better survival. Thus, LNR ≤ 0.16 may be the critical point for determining the better survival prognosis of ANET patients.
ANETs are mostly discovered coincidentally during appendectomy and usually have a benign clinical course. As the major form of appendiceal neoplasms, ANETs are rare appendiceal neoplasms[21]. These tumors are generally confirmed by pathological examination in appendectomy specimens[22]. In the ENETS guidelines, tumor size (including T class), localization within the appendix, extent of invasion into the mesoappendix, and vascular invasion are the main prognostic features. Tumor size, meso-appendiceal invasion, tumor grade, tumor location, and angioinvasion or lymphatic invasion are considered as risk factors that may be associated with disease course and therapy methods[20]. Under some circumstances, RHC should be considered as an additional operation after appendectomy in 3 mo[19,23,24]. The NANET and ENETS guidelines show that tumor size is closely related to the survival, and the prognosis of patients with tumors ≥ 2 cm is worse. Moreover, deep invasion, regional metastasis, and LN metastasis are also related to tumor size[18]. Abdelaal et al[12] reviewed 32 appendectomy specimens that were histologically confirmed as NETs and indicated that appendectomy is an adequate surgical method for patients with tumors smaller than 2 cm with negative pathological margins. Bamboat and Berger[25] reported on five patients with tumors greater than 2 cm and four of the patients were treated by secondary RHC following appendectomy, and they were all alive with a mean follow-up of 10 years (range, 1-15 years). Moertel et al[26] studied 150 patients with ANETs; LN metastasis was observed in 7 (30.43%) of 23 patients with tumors ≥ 2 cm, while no LN metastasis was observed in 123 patients with tumors < 2 cm. Mullen et al[27] reported that LN metastases were present in 44 of 89 patients (49%), including 4 of 27 patients (15%) with tumors ≤ 1.0 cm, 16 of 34 patients (47%) with tumors between 1.0 cm and 2.0 cm, and 24 of 28 patients (86%) with tumors > 2.0 cm, and they concluded that increasing tumor size predicts LN involvement.
Tumor size > 2 cm is the most accepted risk factor, but it remains controversial. According to published data, the cutoff value of tumor size related to LN involvement is 1.55 cm[28]. Rault-Petit et al[29] suggested that 1.95 cm is the optimal cutoff value of tumor size to predict LN status of ANETs. Mehrvarz Sarshekeh et al[16] suggested that 1 cm is a more appropriate cutoff than 2 cm for predicting LN metastasis. Kleiman et al[30] performed a retrospective study of 79 patients and noted that tumors < 2 cm with small-vessel invasion had similar metastatic potential as those ≥ 2 cm. Except, histology is also a significant LN metastasis predictor[31]. Pawa et al[32] suggested that the differentiation grade may be associated with LN metastasis because all G2 and G3 patients have regional LN metastasis. Brighi et al[28] reported that G2 and lymphovascular infiltration are related to LN involvement other than tumor size > 1.55 cm. Carr et al[33] suggested that patients with tumors ≥ 2 cm but with subserosa or mesoappendix invasion, lymphovascular invasion, or increased mitotic activity (> 2 mitoses per 50 high-power fields) are at risk for LN or distant metastasis in some cases. For tumor size and LN metastasis in the present study, patients with tumors > 2 cm had a LN metastasis rate of 39.4% compared to the rate of 6.0% in patients with tumors ≤ 2 cm. The χ2 test showed that there was statistical significance, indicating that tumor size > 2 cm is a factor associated with LN metastasis. At present, there is no factor or rule that completely and accurately predicts LN metastasis. Until additional evidence becomes available, our data analysis combined with the results of most research suggest that tumor larger than 2 cm is still considered to be an important risk factor for LN metastasis.
In terms of treatment, the ENETS guidelines recommended that patients with a tumor diameter > 2 cm should be treated by RHC[20]. However, a substantial number of patients may not receive appropriate surgical resection despite the current treatment recommendations. A population-based retrospective study has suggested that 28% of ANET patients with tumors > 2 cm do not undergo RHC, whereas 3.47% with tumors > 2 cm did not undergo RHC in the present study[34]. For patients with tumors > 2 cm, 96.53% of them underwent colectomy or greater surgery, and 86.71% of patients with tumors ≤ 2 cm underwent colectomy or greater surgery. Thus, these findings suggested that it is not appropriate to perform colectomy or greater surgery only on the basis of tumor size. Grozinsky-Glasberg et al[35] suggested that when using the latest ENETS criteria for RHC, the risk of residual disease is high in patients with a primary tumor size of 1-2 cm, and residual disease may be missed in 18% of ANET patients because pathological factors are ignored. Univariate survival analysis showed that there was a significant difference between patients with tumors > 2 cm and ≤ 2 cm in the present study, but there was no survival difference between the two surgeries stratified according to tumor size. Mehrvarz Sarshekeh et al[16] suggested that differentiation grade and LN involvement are associated with prognosis irrespective of surgery type. Groth et al[31] reported that there is no significant difference in the survival rate between hemicolectomy and appendectomy. Similar results were obtained in our study for patients with tumors ≤ 2 cm and > 2 cm. Colectomy or greater resection did not statistically improve the outcome, but there was a better survival rate for patients with tumors ≤ 2 cm and patients who underwent local resection. Importantly, 74.78% of patients with tumors ≤ 2 cm underwent colectomy or greater resection, indicating that some patients do not undergo proper surgical treatment and that colectomy or greater resection should be strictly applied, especially for those patients with tumors ≤ 2 cm. Volante et al[36] suggested that RHC recommended by the NANET/ENETS guidelines should be followed even though there is no survival difference. Our data analysis showed that when the tumor size was less than 2 cm, there was no survival benefit due to expansion surgery. Therefore, for ANETs less than 2 cm, right hemicolectomy should be carefully selected. According to our analysis results, when the tumor was larger than 2 cm, the two different surgical methods did not show the expected survival difference. However, only 21 patients with tumors larger than 2 cm chose local resection, which may have produced statistical bias. Thus, our findings suggested that it is inappropriate to perform colectomy or larger surgery based only on the size of the tumor. Therefore, we inferred that the survival benefits of the different surgical methods are not due to the choice of surgical methods but instead are due to the difference in the size of the tumor. Because most patients with tumors larger than 2 cm tend to choose colectomy, the prognosis of such patients is inherently worse than that of patients with tumors smaller than 2 cm. Therefore, the observation that patients who choose colectomy has a worse prognosis than those undergoing local resection is probably not caused by the choice of surgical method but by the size and stage of the tumor itself. Combined with the recommendations of guidelines, most studies and our data analysis suggest that patients with tumors larger than 2 cm are more inclined to choose colon resection and that it is unnecessary to blindly expand the scope of surgical resection for patients with tumors ≤ 2 cm.
ANETs are often thought to have good outcomes, and the 10-year survival rate has been reported to reach up to 95%. A previous study has reviewed 83 ANET patients diagnosed during 1976-1987 and indicated that 53 of 56 (94.6%) were alive[13]. A retrospective study has revealed that the 5-year survival rate of 17 patients with ANETs was as high as 100%[9]. A recent retrospective study with a larger sample reported a low CSS rate. In the present study, the survival data indicated that the 10-, 5-, and 3-year CSS rates were 81.7%, 87.5%, and 91.2%, respectively. Moreover, our analysis also calculated survival rates based on disease stage to obtain additional details for the 3- and 5-year CSS rates of patients with disease stages I-IV. The highest 3-year rate was 99.7% for stage I, and the lowest 5-year rate was 25.1% for stage IV.
LN metastasis is often thought to be associated with poor outcomes. Node-positive patients have a significantly worse survival rate even though patients have undergone hemicolectomy and have 12 ELNs[31]. Similar results have been confirmed in another study, which indicated that survival is markedly worse despite RHC being conducted in mixed adenoneuroendocrine carcinoma patients with LN metastasis[16]. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology recommend that 12 LNs should be evaluated at least in colorectal cancer to allow patients to be pathologically assessed accurately and optimally staged based on adequate resected LNs[37]. However, to date, few studies have focused on the impact of the optimal number of ELNs on survival of patients with ANETs. We divided all patients into two groups according to the number of ELNs, and the most significant survival difference existed between patients with ELNs ≤ 10 and those with ELNs ≥ 11. For a certain lymph status, node-negative patients with ELNs ≥ 7 had the most significant survival difference and ≥ 18 for node-positive patients. The optimal number of ELNs may be transformed into LNs and should be surgically retrieved after further confirmation in the future, especially for patients suspiciously diagnosed as having ANETs preoperatively. Except for tumor size, more factors should be taken into account and more detailed criteria should be adopted to choose a surgery type for ANET patients.
The LN status of most malignancies has long been categorized according to the number of metastatic LNs in the AJCC TNM system[38]. However, the number of LNs to be examined often has an influence on the number of metastatic LNs pathologically confirmed. Moreover, the LNR is considered a better prognostic determinant than the number-based LN staging system for colon cancer[39]. The LNR takes both ELNs and positive LNs into account. There is no unified criterion that has been established for LNR stratification of ANETs. The use of quartiles may be the most prevalent method and has been applied in diverse studies. With X-tile software, we adopted 0.16 and 0.48 as cutoff points to divide patients into three groups. The 3-, 5-, and 10-year CSS rates significantly increased with a lower ratio (≤ 0.16). To some extent, the present study agreed with the study by Vaccaro et al[40], who found that colon cancer patients with an LNR < 0.25 have better survival. Lee et al[39] also suggested that an LNR < 0.11 is associated with a significantly better 5-year disease-free survival. Shinto et al[41] mentioned that patients with a low LNR have a higher 5-year CSS rate; the LNR cutoff is 0.18 for all colon cancer patients and 0.16 and 0.22 for right and left colon cancer patients, respectively. The LNR cutoff of ANETs in the present study was similar to the values proposed by other studies. For node-positive patients, LNR ≤ 0.16 increased the 3-, 5-, and 10-year CSS rates from 67.3%, 58.4%, and 48.9% to 88.5%, 80.8%, and 68.9%, respectively. Our analysis results suggested that higher LNR results in a worse survival prognosis. Thus, LNR ≤ 0.16 may be the critical point for determining a better survival of ANET patients.
In summary, the univariate survival analysis conducted in the present study showed that most factors are related to survival. Patients with tumor size > 2 cm are more likely to develop LN invasion and metastasis with a worse prognosis. Regarding the choice of surgical methods, for patients with tumors ≤ 2 cm, there is no need to blindly expand the scope of surgical resection. Higher positive rate of LN metastasis in patients with ANETs result in a worse survival prognosis. The optimal number of ELNs for all patients, node-negative patients, and node-positive patients is 11, 7, and 18, respectively. LNR ≤ 0.16 may be the key point for determining a better survival prognosis of patients with ANETs.
Appendiceal neuroendocrine tumors are often confirmed by pathological examination after appendicectomy. It is unclear how many lymph nodes should be surgically removed for neuroendocrine tumors occurring in the appendix so that the patients could achieve a better survival.
Detailed survival rates of patients with appendiceal neuroendocrine tumors are not clear, especially for those with different disease stages and lymph statuses. The relationship between different numbers of examined lymph nodes and survival rates for appendiceal neuroendocrines tumor has not been described.
With data of 4583 patients with appendiceal neuroendocrine tumors, the study aimed to describe factors that could have an effect on patients survival and survival rates for different disease stages, to verify whether it is reliable to choose surgery type only according to tumor size and the relationship between tumor size and lymph metastasis, and to determine the optimal number of examined lymph nodes and the optimal lymph node positive rate for patients with appendiceal neuroendocrine tumors.
This retrospective study included patients with appendiceal neuroendocrine tumors who underwent surgical resection in the SEER database. The clinical characteristics were described. X-tile software was used to determine the optimal cutoff points. Cancer-specific survival curves were plotted using the Kaplan–Meier method and survival differences were estimated by the log-rank test.
Blindly expanding the scope of surgical resection did not bring survival benefits. There were optimal cutoff points of examined lymph nodes and lymph node positive rate that could bring a better survival.
The optimal numbers of examined lymph nodes are different according to lymph node status.
More appendiceal neuroendocrine patients with tumors larger than 2 cm but undergoing local resection can be contrasted to those undergoing colectomy or greater resection in future. The optimal values of examined lymph nodes and lymph node positive rate can be further determined if more factors are taken into account.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Research and experimental medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Turkey; Cabezuelo AS, Spain S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Li X
| 1. | Nilsson O. Gastrointestinal carcinoids--aspects of diagnosis and classification. APMIS. 1996;104:481-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, Liu W, Zhao Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018;45:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 1750] [Article Influence: 437.5] [Reference Citation Analysis (2)] |
| 4. | Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, Chen QY, Lin M, Tu RH, Huang CM. Incidence and survival trends for gastric neuroendocrine neoplasms: An analysis of 3523 patients in the SEER database. Eur J Surg Oncol. 2018;44:1628-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 502] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 6. | Kelly KJ. Management of Appendix Cancer. Clin Colon Rectal Surg. 2015;28:247-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Şenel F, Karaman H, Demir H. Neuroendocrine tumors detected in appendectomy specimens: ten-year single-center experience. Turk J Med Sci. 2018;48:68-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Akbulut S, Tas M, Sogutcu N, Arikanoglu Z, Basbug M, Ulku A, Semur H, Yagmur Y. Unusual histopathological findings in appendectomy specimens: a retrospective analysis and literature review. World J Gastroenterol. 2011;17:1961-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 79] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Kocaöz S, Turan G. Assessment of appendix carcinoid tumors: A retrospective study. Indian J Pathol Microbiol. 2019;62:413-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB; UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 11. | Man D, Wu J, Shen Z, Zhu X. Prognosis of patients with neuroendocrine tumor: a SEER database analysis. Cancer Manag Res. 2018;10:5629-5638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 90] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 12. | Abdelaal A, El Ansari W, Al-Bozom I, Khawar M, Shahid F, Aleter A, Abunuwar MR, El-Menyar A. Frequency, characteristics and outcomes of appendicular neuroendocrine tumors: A cross-sectional study from an academic tertiary care hospital. Ann Med Surg (Lond). 2017;21:20-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Shaw JH, Canal A. Carcinoid tumours in Auckland, New Zealand. Aust N Z J Surg. 1989;59:229-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Guzman C, Boddhula S, Panneerselvam N, Dodhia C, Hellenthal NJ, Monie D, Monzon JR, Kaufman T. Appendiceal Carcinoid Tumors: Is There a Survival Advantage to Colectomy over Appendectomy? J Gastrointest Surg. 2020;24:1149-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG. Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. Ann Oncol. 2001;12:1295-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Mehrvarz Sarshekeh A, Advani S, Halperin DM, Conrad C, Shen C, Yao JC, Dasari A. Regional lymph node involvement and outcomes in appendiceal neuroendocrine tumors: a SEER database analysis. Oncotarget. 2017;8:99541-99551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Kano K, Yamada T, Yamamoto K, Komori K, Watanabe H, Hara K, Shimoda Y, Maezawa Y, Fujikawa H, Aoyama T, Tamagawa H, Yamamoto N, Cho H, Shiozawa M, Yukawa N, Yoshikawa T, Morinaga S, Rino Y, Masuda M, Ogata T, Oshima T. Association Between Lymph Node Ratio and Survival in Patients with Pathological Stage II/III Gastric Cancer. Ann Surg Oncol. 2020;27:4235-4247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Boudreaux JP, Klimstra DS, Hassan MM, Woltering EA, Jensen RT, Goldsmith SJ, Nutting C, Bushnell DL, Caplin ME, Yao JC; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39:753-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 19. | Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R, Grossman A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95:135-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, Krenning E, Reed N, O'Toole D; Vienna Consensus Conference participants. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Galanopoulos M, Toumpanakis C. The Problem of Appendiceal Carcinoids. Endocrinol Metab Clin North Am. 2018;47:661-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kunduz E, Bektasoglu HK, Unver N, Aydogan C, Timocin G, Destek S. Analysis of Appendiceal Neoplasms on 3544 Appendectomy Specimens for Acute Appendicitis: Retrospective Cohort Study of a Single Institution. Med Sci Monit. 2018;24:4421-4426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 82] [Cited by in F6Publishing: 94] [Article Influence: 23.5] [Reference Citation Analysis (6)] |
| 24. | Toumpanakis C, Fazio N, Tiensuu Janson E, Hörsch D, Pascher A, Reed N, O Apos Toole D, Nieveen van Dijkum E, Partelli S, Rinke A, Kos-Kudla B, Costa F, Pape UF, Grozinsky-Glasberg S, Scoazec JY; The ENETS 2016 Munich Advisory Board Participants; ENETS 2016 Munich Advisory Board Participants. Unmet Needs in Appendiceal Neuroendocrine Neoplasms. Neuroendocrinology. 2019;108:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Bamboat ZM, Berger DL. Is right hemicolectomy for 2.0-cm appendiceal carcinoids justified? Arch Surg. 2006;141:349-52; discussion 352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Moertel CG, Weiland LH, Nagorney DM, Dockerty MB. Carcinoid tumor of the appendix: treatment and prognosis. N Engl J Med. 1987;317:1699-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 232] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Mullen JT, Savarese DM. Carcinoid tumors of the appendix: a population-based study. J Surg Oncol. 2011;104:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Brighi N, La Rosa S, Rossi G, Grillo F, Pusceddu S, Rinzivillo M, Spada F, Tafuto S, Massironi S, Faggiano A, Antonuzzo L, Santini D, Sessa F, Maragliano R, Gelsomino F, Albertelli M, Vernieri C, Panzuto F, Fazio N, De Divitiis C, Lamberti G, Colao A, Fave GD, Campana D. Morphological Factors Related to Nodal Metastases in Neuroendocrine Tumors of the Appendix: A Multicentric Retrospective Study. Ann Surg. 2020;271:527-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Rault-Petit B, Do Cao C, Guyétant S, Guimbaud R, Rohmer V, Julié C, Baudin E, Goichot B, Coriat R, Tabarin A, Ramos J, Goudet P, Hervieu V, Scoazec JY, Walter T. Current Management and Predictive Factors of Lymph Node Metastasis of Appendix Neuroendocrine Tumors: A National Study from the French Group of Endocrine Tumors (GTE). Ann Surg. 2019;270:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Kleiman DA, Finnerty B, Beninato T, Zarnegar R, Nandakumar G, Fahey TJ 3rd, Lee SW. Features Associated With Metastases Among Well-Differentiated Neuroendocrine (Carcinoid) Tumors of the Appendix: The Significance of Small Vessel Invasion in Addition to Size. Dis Colon Rectum. 2015;58:1137-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Groth SS, Virnig BA, Al-Refaie WB, Jarosek SL, Jensen EH, Tuttle TM. Appendiceal carcinoid tumors: Predictors of lymph node metastasis and the impact of right hemicolectomy on survival. J Surg Oncol. 2011;103:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Pawa N, Clift AK, Osmani H, Drymousis P, Cichocki A, Flora R, Goldin R, Patsouras D, Baird A, Malczewska A, Kinross J, Faiz O, Antoniou A, Wasan H, Kaltsas GA, Darzi A, Cwikla JB, Frilling A. Surgical Management of Patients with Neuroendocrine Neoplasms of the Appendix: Appendectomy or More. Neuroendocrinology. 2018;106:242-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Carr NJ, Emory TS, Sobin LH. Chapter 24 - Epithelial Neoplasms of the Appendix. In: Odze RD and Goldblum JR (ed.). Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas (Second Edition). W.B. Saunders, Philadelphia. 2009: p639-652. [Cited in This Article: ] |
| 34. | McGory ML, Maggard MA, Kang H, O'Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264-2271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Grozinsky-Glasberg S, Alexandraki KI, Barak D, Doviner V, Reissman P, Kaltsas GA, Gross DJ. Current size criteria for the management of neuroendocrine tumors of the appendix: are they valid? Neuroendocrinology. 2013;98:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Volante M, Daniele L, Asioli S, Cassoni P, Comino A, Coverlizza S, De Giuli P, Fava C, Manini C, Berruti A, Papotti M. Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: a retrospective clinical pathologic analysis of 138 cases. Am J Surg Pathol. 2013;37:606-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Engstrom PF, Arnoletti JP, Benson AB 3rd, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, Fakih MG, Fleshman J Jr, Fuchs C, Grem JL, Kiel K, Knol JA, Leong LA, Lin E, Mulcahy MF, Rao S, Ryan DP, Saltz L, Shibata D, Skibber JM, Sofocleous C, Thomas J, Venook AP, Willett C; National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 38. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6157] [Article Influence: 439.8] [Reference Citation Analysis (0)] |
| 39. | Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, Kim C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Vaccaro CA, Im V, Rossi GL, Quintana GO, Benati ML, Perez de Arenaza D, Bonadeo FA. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum. 2009;52:1244-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Shinto E, Ike H, Hida JI, Kobayashi H, Hashiguchi Y, Kajiwara Y, Hase K, Ueno H, Sugihara K. Marked impact of tumor location on the appropriate cutoff values and the prognostic significance of the lymph node ratio in stage III colon cancer: a multi-institutional retrospective analysis. J Gastroenterol. 2019;54:597-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |