Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.870
Peer-review started: August 18, 2021
First decision: October 3, 2021
Revised: October 19, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
The incidence of gastric cancer is high. The number of dissected lymph nodes was an independent factor affecting prognosis. Although preoperative labeling is helpful in lymph nodes resection, there are no guidelines for when to perform preoperative labeling.
To investigate the role of nanocarbon in lymph node dissection during gastrectomy, and to discuss the relationship between the timing of preoperative injection of carbon nanoparticles and the extent of lymph node dissection.
A prospective analysis was performed on the clinical data of 307 patients with advanced gastric cancer who underwent laparoscopic surgery in the General Surgery Department of Weifang People’s Hospital between June 2018 and February 2021. The patients were randomly divided into experimental group and control group based on whether they received preoperative nanocarbon injection or not. The experimental group was divided into different groups according to the preoperative labeling time. The number of dissected lymph nodes and the number of lymph nodes with black staining were compared in each group after surgery, and the role of nanocarbon in the number of dissected lymph nodes, pathological staging, and the relationship with prognosis were discussed.
The average number of dissected lymph nodes in the experimental group was higher than that in the control group. In the experimental group, the number of lymph node dissections and number of black-staining lymph nodes in the nanocarbon-labeling group at 2 d and 1 d before surgery were higher than in the labeling group on the day before surgery (P < 0.05).
Preoperative nanocarbon labeling can safely and effectively guide lymph node dissection. To improve the detection rate of lymph nodes is conducive to subsequent comprehensive anti-tumor therapy.
Core Tip: This study investigated the role of carbon nanoparticles in lymph node dissection during gastrectomy, and discussed the relationship between the timing for preoperative labeling and the number of lymph nodes dissected. It was found that carbon nanoparticle labeling has a role in guiding laparoscopic lymph node dissection of gastric cancer. Preoperative submucosal injection of carbon nanoparticles could significantly improve the detection rate of lymph nodes, which is conducive to pathological staging and subsequent comprehensive antitumor therapy.
- Citation: Zhao K, Shan BQ, Gao YP, Xu JY. Role of carbon nanotracers in lymph node dissection of advanced gastric cancer and the selection of preoperative labeling time. World J Clin Cases 2022; 10(3): 870-881
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/870.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.870
Stomach cancer is the fifth most common cancer worldwide and the third leading cause of cancer mortality. There are significant regional differences in the incidence of gastric cancer. The incidence in Asia is significantly higher than that in Europe and the United States. Gastric cancer deaths in China account for > 40% of the global total in the same period[1]. Reducing cancer-related mortality and improving quality of life is one of the current research focuses.
Radical surgery is still the preferred treatment method for advanced gastric cancer. Improvements in technology have resulted in laparoscopy becoming the preferred surgical method at present, which has obvious advantages over open surgery in terms of surgical field and number of lymph nodes dissected. Previous studies[2-4] have shown that there is a correlation between the number of dissected lymph nodes and prognosis. The staging method based on the number of lymph node metastases in gastric cancer (pN staging) is currently recognized as the best staging method for lymph node metastasis in gastric cancer. Postoperative lymph node detection rate is one of the major factors affecting pN staging of lymph node metastasis after radical gastrectomy[5]. To help surgeons correctly distinguish the normal tissue from the lymph nodes and dissect lymph nodes as much as possible[6], markers can be injected around the tumor to stain the lymph nodes. Currently, the most commonly used lymphatic tracer is indocyanine green (ICG), whose mechanism of action is as follows: (1) After injection around the tumor, some ICG binds to albumin in the tissue and remains locally, therefore the tumor is colored under fluorescent laparoscopy, some of the ICG gradually combines with albumin in lymphatic vessels and drains to the lymph nodes, finally returning to the blood circulation, and metabolizes in the liver[6]. The slow flow rate of lymphatic fluid and the presence of lymph nodes make the lymphatic system slow to transport ICG, so ICG can persist in the lymphatic system for a long time, and this is how ICG is applied to lymph node dissection, but the application of ICG requires special fluorescence laparoscopy; (2) Methylene blue is a lymphatic contrast agent that is easy to prepare and inject. After injection, methylene blue enters the lymphatic and blood capillaries, quickly turning the surrounding tissues blue, but its diffusion is too fast, so lymph nodes must be identified and removed quickly[7]. Therefore, due to its rapid diffusion, some lymph nodes may be missed; and (3) Nanocarbon suspension injection, as a new type of tracer, has stable physical and chemical properties and a strong affinity for lymphatic tissue. The nanometer carbon lymph tracer tag is injected near the tumor tissue. Within a short time, it is taken up by the macrophages, accumulated in the lymph vessels, and remains in the lymph nodes due to its high lymphoid affinity. The lymph nodes are stained black, which serves the purpose of staining the draining lymph nodes near the tumor tissue[8].
In this study, preoperative endoscopic injection of carbon nanoparticles was selected due to the obvious contrast after the labeling of carbon nanoparticles, longer tissue fixation time, high lymph node affinity, and lack of need for special instruments and other characteristics. In gastric cancer and other gastrointestinal malignancies, there are still no specific guidelines for when preoperative labeling should be performed. On the basis of previous research, we extended the time of preoperative submucosal tracer injection, and the experimental group was divided into three: operation day (2-6 h) labeling group, preoperative 1-d (18-24 h) labeling group and preoperative 2-d (42-48 h) labeling group. The differences in the number of lymph nodes detected and the number of black-staining lymph nodes between the three groups and the control group were compared. The results provide direction for further research.
We prospectively analyzed 307 patients with advanced gastric cancer who were hospitalized for surgery in the Department of General Surgery of Weifang People’s Hospital between June 2018 and February 2021. According to the different primary location of the tumor in the stomach, laparoscopic subtotal gastrectomy (LSG) or laparoscopic total gastrectomy (LTG) was selected, and D2 Lymph node dissection was performed for all patients. The lymph nodes that needed to be dissected for different surgical procedures are shown in Table 1. There were 180 patients in the experimental group and 127 in the control group. To determine the preoperative labeling time, patients were randomly allocated to receive endoscopic labeling with nanocarbon suspension in the experimental group on the day of surgery, 1 d before surgery, and 2 d before surgery. The control group was not labeled with nanocarbon and the other treatment measures were the same.
| Lymphnodes dissection extent | Lymphadenectomy |
| LTG | |
| D1 | No. 1-7 |
| D2 | D1 + No. 8a, 9, 10, 11p, 11d, 12a |
| LSG | |
| D1 | No. 1, 3, 4sb, 4d, 5, 6, 7 |
| D2 | D1 + No. 8a, 9, 11p, 12a |
The inclusion criteria were: (1) Signed informed consent was given for surgery; (2) Gastroscopic examination and pathological biopsy confirmed malignant tumor of the stomach; (3) Abdominal enhanced computed tomography or magnetic resonance imaging and other ancillary examinations showed no distant metastasis; (4) No important organ dysfunction and could tolerate surgery; and (5) Clinical stage advanced gastric cancer.
The exclusion criteria were: (1) Secondary examination suggested multiple metastases without surgical indications; (2) Patients with important organ dysfunction or other major diseases and could not tolerate surgery; and (3) Other contraindications and could not undergo D2 radical surgery.
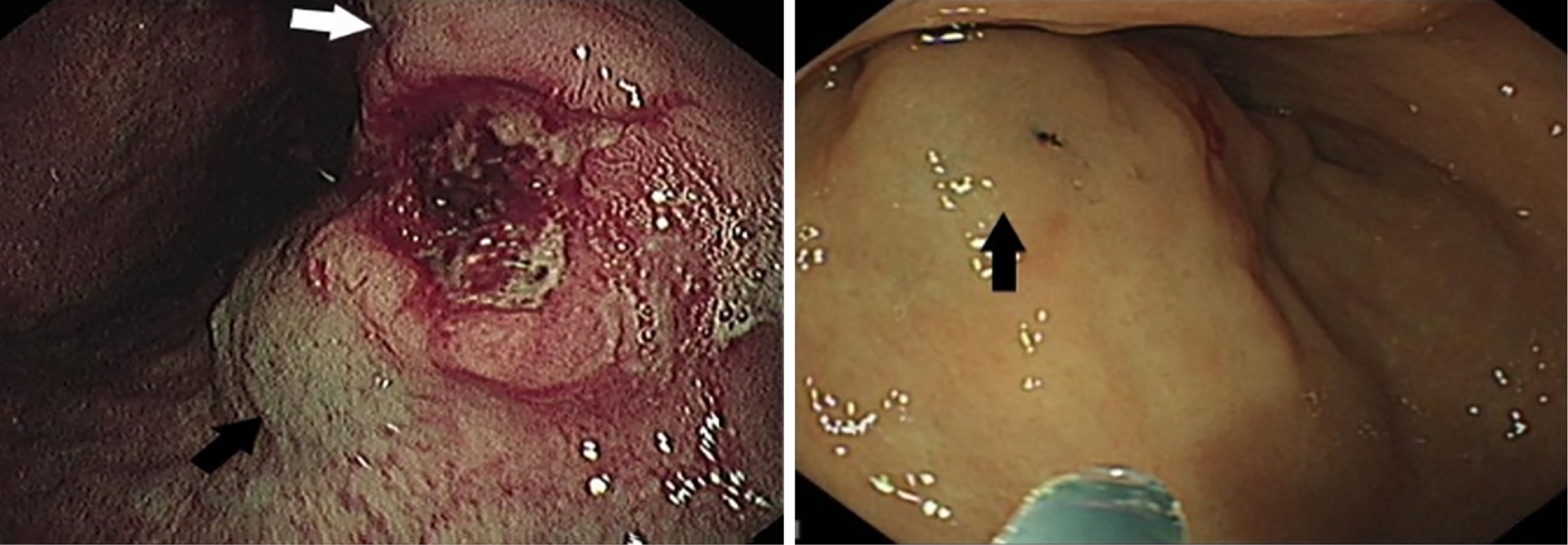
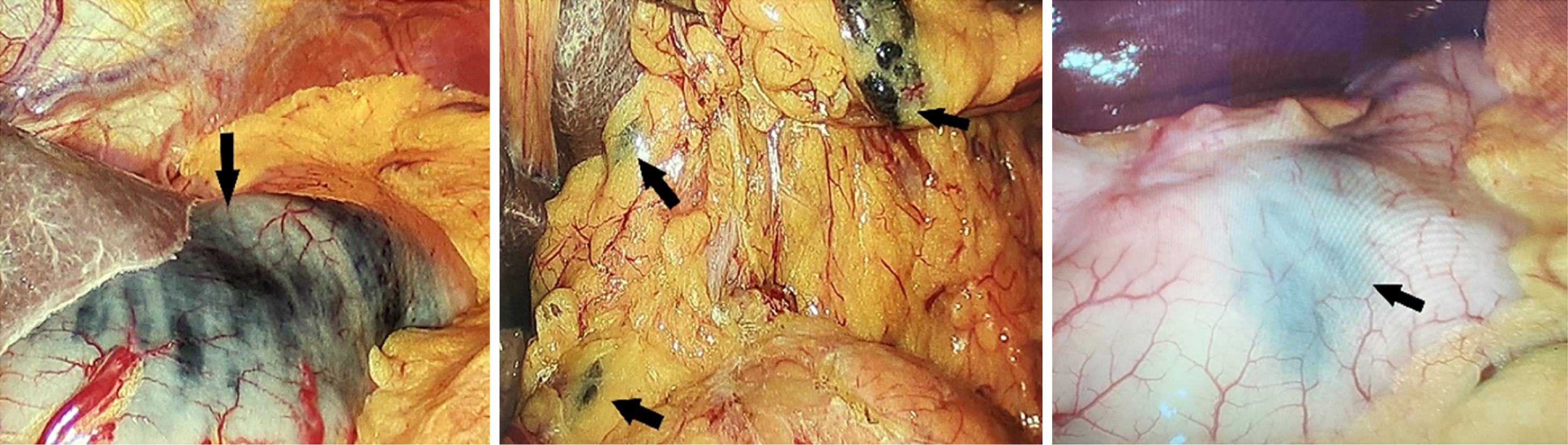
When performing the preoperative nanocarbon labeling, if the submucosal injection level is too shallow, the nanocarbon could not enter the lymphatic flow to mark cancer tissue, and if the injection level is too deep, the nanocarbon would penetrate the serosal membrane and pollute the surgical field of view. Therefore, the sandwich labeling method was adopted: and 2-4 points were selected at 0.5-1.0 cm from the tumor edge. Normal saline was first injected to raise the submucosa, nanocarbon was injected into the submucosal surface, and subsequently, normal saline was injected again to increase the pressure of submucosal carbon nanoparticles suspension, so that the carbon nanoparticles could easily penetrate into the lymphatic tissues. A total of 2.0 mL nanocarbon suspension was injected. Radical proximal gastric cancer resection was not considered, and all patients were labeled at the oral but not at the anal end. Figure 1 shows preoperative carbon nanoparticles injection, and Figure 2 shows intraoperative black staining of lymph nodes and surrounding tissues.
All 307 patients received standard gastric cancer resection and D2 Lymph node dissection. According to different gastric cancer locations, 176 patients underwent LTG and 131 patients underwent LSG. LTG lymph node dissection and anastomosis procedures were as follows: The gastrocolonic ligament was dissected along the transverse colon, the right gastroomentum vessel was ligated at the root, and No. 6 Lymph nodes were dissected. The anterior lobe of the hepatoduodenal ligament was separated and resected, the right gastric vessel was severed and ligated, and No. 5 and 12 Lymph nodes were dissected. Duodenum was severed from the lower duodenal bulb of pylorus with a linear stapler. The stomach was turned to the left upper abdomen, the left gastric vessels were ligated at the root and cut off, and No. 7, 8, 9, and 11 Lymph nodes were dissected. The hepatogastric ligament was opened, and the No. 1 Lymph nodes were dissected along the right side of cardia. The greater curvature omentum was removed, the left gastro-omental artery was ligated at the root, the spleen and stomach ligaments and the posterior gastric artery were severed, and the No. 2, 4, and 10 Lymph nodes were dissected. The Esophagojejunal (π) anastomosis and Braun anastomosis were performed.
LSG lymph node[M1] dissection and anastomosis procedures were: The loose connective tissue between the anterior and posterior lobes of the right transverse mesocolon was extended gradually to the left until the first short gastric vessel behind the root of the left gastroomentum artery. The No. 6 Lymph nodes were dissected from the right omentum vessel. Hepatoduodenal ligament was opened, and No. 5, and 12a lymph nodes were dissected along the proper hepatic artery. The root of the right gastric artery was exposed and along the main trunk of the right gastric artery, the surrounding soft tissue was dissected, the left gastric artery and the beginning part of the coronary vein were exposed, the root of the vessel was ligated, and the surrounding No. 7, 8a, 9, and 11p lymph nodes were dissected. Mesangial tissue was isolated along the lesser curvature of the stomach till the right diaphragm, and No. 1, 3a, 3b and 5 Lymph nodes were dissected. Billroth II and Braun anastomosis was performed. All procedures were performed by the same group of surgeons.
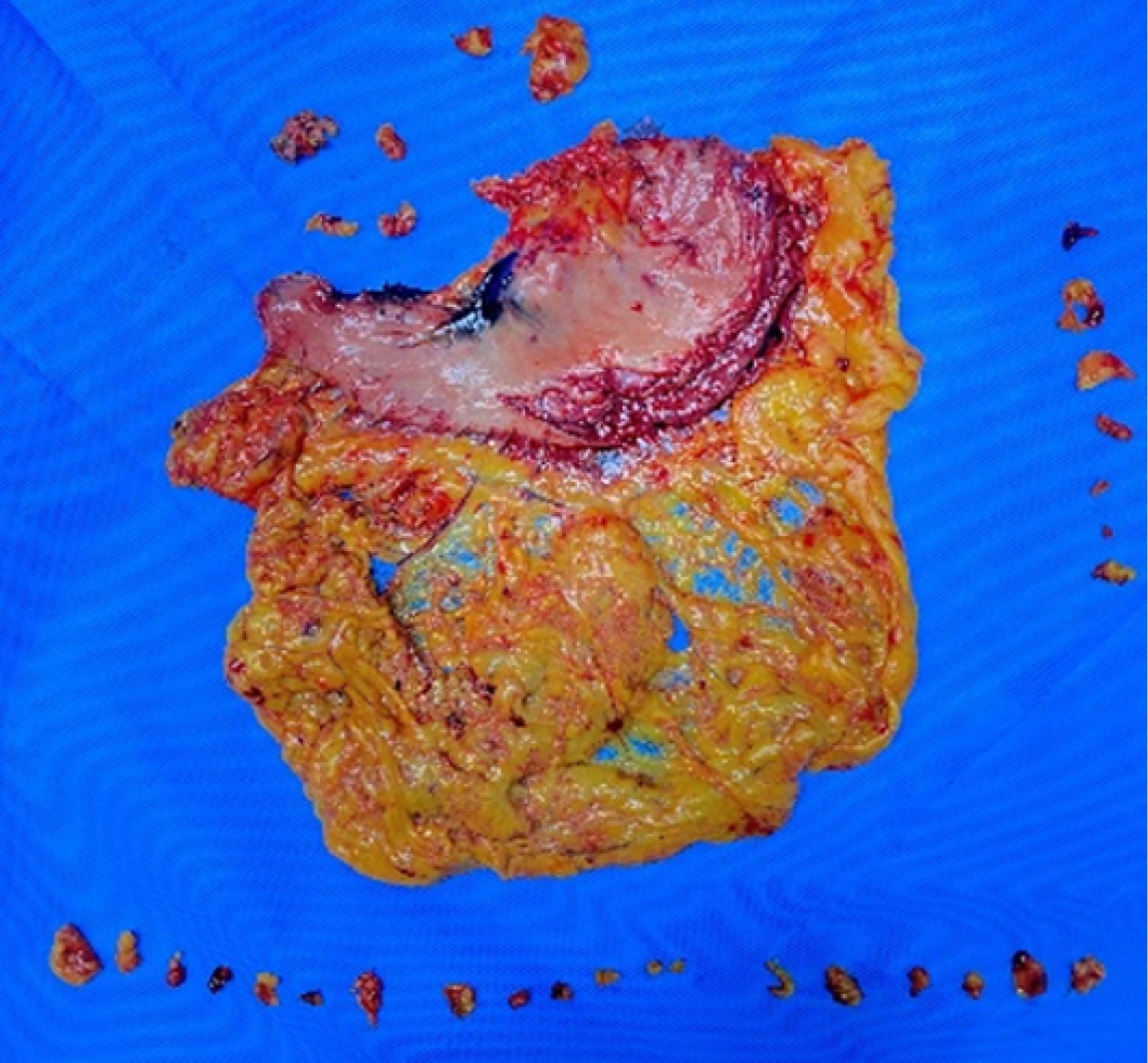
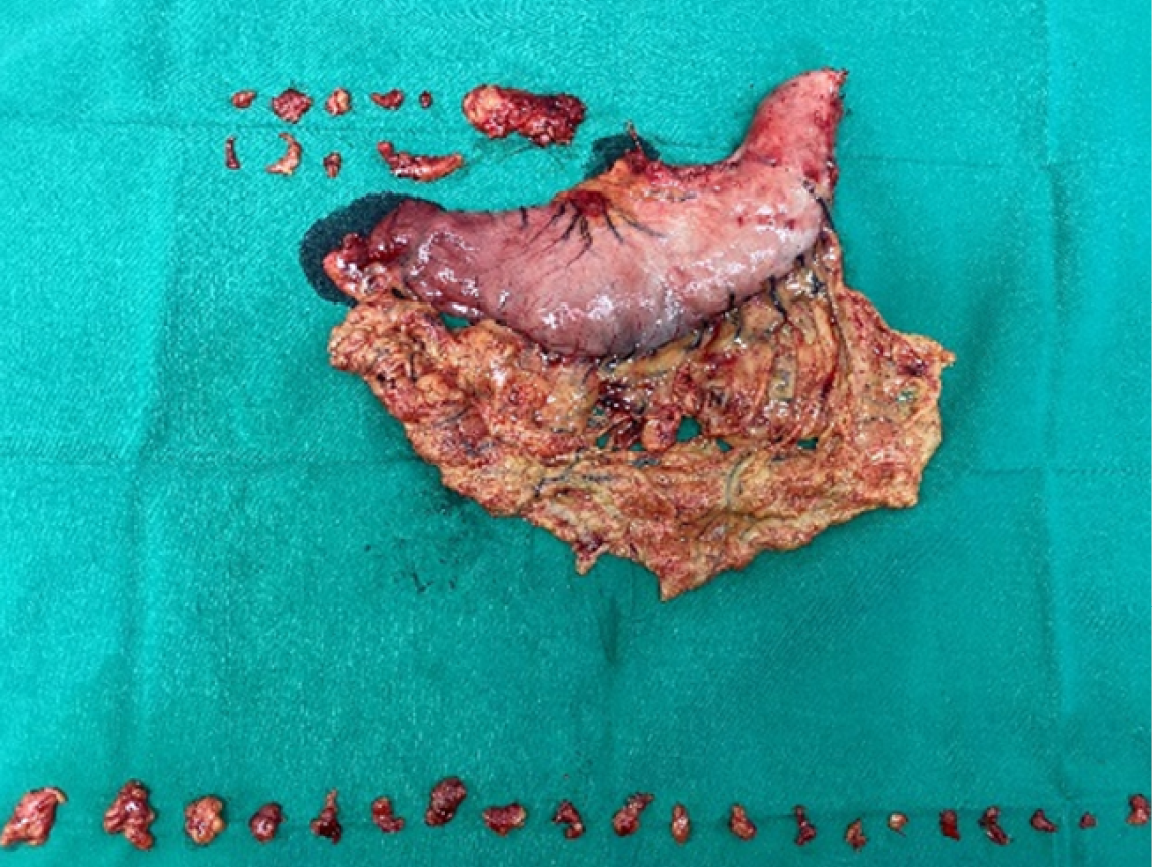
After the surgical specimen was isolated, the senior attending physician placed it according to its anatomical position and took photographs. The lymph nodes in each group around the stomach were cut and marked according to the blood vessels. The tissues in each group were finely separated and the surface tissues of lymph nodes were removed, and bagged separately. All lymph nodes were sent to the pathology department for postoperative analysis according to their corresponding perigastric lymph node groups. For the patients with total gastrectomy, the gastric peripheral lymph nodes on the lower cardia side were classified as No. 1, the gastric peripheral lymph nodes on the greater cardia side were classified as No. 2, and the peripheral tissues of short gastric vessels above the left arteriovenous Hemlok clip were classified as No. 4sa. The left arteriovenous clipped tissue along the gastric omentum was classified as No. 4sb, the right arteriovenous clipped tissue along the gastric omentum was classified as No. 4d, and the subpyloric region was classified as No. 6. Ligation of the right gastric arteriovenous Hemlok clipped to the upper part of the pylorus was classified as No. 5, from the ligation of the left gastric arteriovenous Hemlok clipped to its first branch was classified as No. 7, and the remaining perigastric tissue near the lesser curvature was classified as No. 3. Figures 3 and 4 shows lymph node sorting after gastric cancer.
The number of dissected lymph nodes, black-stained lymph nodes were counted, and basic information, including gender, age, pathological types, postoperative complications such as intraoperative blood loss, and anastomotic fistula, were observed.
SPSS 25.0 was used for statistical analysis. Measurement data were expressed as mean ± SD, and a t test was used for comparison between the two groups. One-way analysis of variance and multiple comparisons were used for intragroup comparison, and P < 0.05 was considered statistically significant.
A total of 307 patients were enrolled. Gender, age, pathological type, pathological stage, tumor markers, intraoperative blood loss (significant), postoperative complications and other indicators are shown in Table 2, and the differences were not significant.
| Basic information | Control group | Experimental group | P value | ||
| LTG | LSG | LTG | LSG | ||
| Number of cases | 77 | 50 | 99 | 81 | |
| Gender | > 0.05 | ||||
| Male | 44 | 27 | 49 | 45 | |
| Female | 33 | 23 | 50 | 36 | |
| Age, yr | 60 ± 17 | 64 ± 18 | > 0.05 | ||
| Pathological types | > 0.05 | ||||
| Highly differentiated adenocarcinoma | 14 | 10 | 14 | 12 | |
| Moderately differentiated adenocarcinoma | 23 | 13 | 30 | 25 | |
| Poorly differentiated adenocarcinoma | 29 | 19 | 45 | 40 | |
| Other types | 11 | 8 | 10 | 4 | |
| T stage | > 0.05 | ||||
| 1 | 10 | 10 | 6 | 4 | |
| 2 | 20 | 11 | 33 | 27 | |
| 3 | 37 | 22 | 44 | 39 | |
| 4 | 10 | 7 | 16 | 11 | |
| N stage | > 0.05 | ||||
| 1 | 15 | 6 | 22 | 13 | |
| 2 | 29 | 20 | 40 | 30 | |
| 3 | 33 | 24 | 37 | 38 | |
| CEA | > 0.05 | ||||
| Normal | 44 | 20 | 42 | 32 | |
| Increased | 33 | 30 | 57 | 49 | |
| CA199 | > 0.05 | ||||
| Normal | 43 | 24 | 50 | 34 | |
| Increase | 34 | 26 | 49 | 47 | |
| Intraoperative blood loss (mL) | 71.13 ± 21.33 | 90.70 ± 31.77 | 61.53 ± 20.38 | 75.69 ± 20.18 | < 0.05 |
| Complication | 3 | 0 | 2 | 2 | > 0.05 |
| Postoperative bleeding | 2 | 0 | 0 | 0 | |
| Anastomotic fistula | 1 | 0 | 1 | 2 | |
| Obstruction | 0 | 0 | 1 | 0 | |
| Postoperative hospital stay (d) | 6.55 ± 4.63 | 7.23 ± 4.51 | 6.54 ± 4.16 | 7.21 ± 4.32 | > 0.05 |
There were 180 patients in the experimental group (99 treated with LTG and 81 with LSG), and a total of 6105 Lymph nodes (3460 LTG and 2645 LSG) were detected, with an average of 34.95 ± 4.81/case for LTG and 32.65 ± 3.82/case for LSG. There were 127 patients in the control group (77 treated with LTG and 50 with LSG), and a total of 4000 Lymph nodes (2456 LTG and 1544 LSG) were detected, with an average of 31.90 ± 4.47/case for LTG and 30.88 ± 2.69/case for LSG (Tables 3 and 4). The differences in the number of dissected lymph nodes, and number of black-stained lymph nodes at D1 and D2 stations under different surgical methods and different preoperative labeling times are shown in Tables 5 and 6.
| Cases | Dissected lymph nodes of D1 station | Average | Statistic difference | ||
| T | P value | ||||
| LTG | |||||
| Experimental group | 99 | 2088 | 19.65 ± 3.08 | 3.066 | 0.003 |
| Control group | 77 | 1590 | 21.09 ± 3.08 | ||
| LSG | |||||
| Experimental group | 81 | 1622 | 20.02 ± 2.69 | 1.700 | 0.091 |
| Control group | 50 | 965 | 19.30 ± 1.72 | ||
| Cases | Dissected lymph nodes of D2 station | Average | Statistic difference | ||
| T | P value | ||||
| LTG | |||||
| Experimental group | 99 | 1372 | 13.85 ± 2.26 | 5.059 | 0.000 |
| Control group | 77 | 943 | 12.25 ± 2.06 | ||
| LSG | |||||
| Experimental group | 81 | 1023 | 12.63 ± 2.22 | 2.855 | 0.005 |
| Control group | 50 | 579 | 11.58 ± 1.73 | ||
| Labeling time (d) | Cases | Average number of dissected lymph nodes | Average number of black staining lymph nodes | |
| LTG | 0 | 30 | 18.87 ± 2.78 | 11.30 ± 2.42 |
| 1 | 36 | 21.86 ± 2.39 | 14.00 ± 2.29 | |
| 2 | 33 | 22.27 ± 2.93 | 14.36 ± 2.45 | |
| P | 0.000 | 0.000 | ||
| F | 14.385 | 14.946 | ||
| LSG | 0 | 16 | 18.19 ± 2.01 | 8.15 ± 1.65 |
| 1 | 34 | 21.03 ± 3.00 | 10.82 ± 2.55 | |
| 2 | 31 | 19.87 ± 1.98 | 10.81 ± 1.94 | |
| P | 0.002 | 0.000 | ||
| F | 7.075 | 9.500 |
| Labeling time (d) | Cases | Average number of dissected lymph nodes | Average number of black staining lymph nodes | |
| LTG | 30 | 12.27 ± 2.46 | 3.07 ± 1.09 | 30 |
| 36 | 14.47 ± 1.64 | 4.14 ± 1.46 | 36 | |
| 33 | 14.64 ± 1.88 | 4.45 ± 1.28 | 33 | |
| 0.000 | 0.000 | |||
| 13.262 | 9.522 | |||
| LSG | 0 | 16 | 11.50 ± 2.12 | 2.75 ± 1.15 |
| 1 | 34 | 12.53 ± 2.39 | 4.74 ± 1.85 | |
| 2 | 31 | 13.32 ± 1.71 | 4.42 ± 1.64 | |
| P | 0.024 | 0.001 | ||
| F | 3.891 | 7.906 |
Over the past 20 years, with the progression of medical science, the comprehensive treatment of gastric cancer has made great strides. Surgical treatment is still the most used method, and lymph node dissection is one of the most important techniques in radical gastrectomy. How to remove a sufficient number of lymph nodes in gastric cancer surgery more safely and effectively to achieve the goal of radical resection has been one of the topics studied by gastrointestinal surgeons. For advanced gastric cancer, the surgical criteria were D2 or D2+ radical surgery: tumor resection and regional lymph node dissection. The metastasis of gastric cancer is mainly via the lymphatic pathway, so survival depends not only on the primary lesion but also on the presence of regional lymph node metastasis[9]. Baxter et al[10] found that a certain number of lymph nodes should be dissected during radical gastrectomy for gastric cancer, and there was a correlation between the number of lymph nodes dissected and prognosis. According to the clinical data of gastric cancer patients in the American Surveillance, Epidemiology, and End Results database[11,12], prognosis can be improved by the addition of 10 Lymph nodes in postoperative specimens. Even for patients with negative lymph nodes after surgery, the number of detected lymph nodes is still an independent factor affecting prognosis[12]. Some researchers have reported that, in order to improve the accuracy of pathological lymph node staging of gastric cancer specimens, at least 10-15 Lymph nodes should be detected in the N0 stage, and ≥ 20 should be detected in the N1-3 stage. If ≥ 30 Lymph nodes are collected for examination, postoperative lymph node staging could be more accurate[13,14]. The number of dissected lymph nodes recommended by the 8th Edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) in TNM staging of gastric cancer should not be less than 16[15]. According to the Japanese Regulations on the Management of Gastric Cancer[16], the number of lymph nodes dissected during radical gastrectomy for gastric cancer should be ≥ 15, and an insufficient number of dissected lymph nodes will significantly affect the 5-year postoperative survival rate[17-19]. The more lymph nodes sent for examination, the greater the possibility of detection of metastatic lymph nodes[20]. The more reliable the accuracy of lymph node staging is, the more the occurrence of lymph node staging migration can be reduced or avoided[21]. Therefore, effective lymph node dissection is indispensable for thorough radical treatment of gastric cancer. According to the multi-center analysis of postoperative gastric cancer survival data[22], there was an obvious postoperative lymph node stage migration phenomenon in Chinese patients with gastric cancer, especially in early-stage patients with < 15 Lymph nodes dissected and in advanced patients with < 35 Lymph nodes dissected. According to a study by Sano et al[17] in 2017, the average number of lymph nodes detected in each gastric cancer specimen in Japan reached 39.4/case, followed by 33.0/case in South Korea, while the figure for several major centers included in the survey in China was only 24.8/case–even lower than the 29.5/case in Europe and America[23].
In order to correctly distinguish between lymph node and normal tissue and to dissect the lymph nodes more thoroughly, we could selectively label the pericancerous lymph nodes. The existing lymphatic tracers can be divided into three generations: the first is represented by methylene blue and India ink, the second by iodine oil and activated carbon, and the third by nanocarbon. Nanocarbon lymph node tracers are essentially lymphatic tracers, and their physical and chemical properties have been described in the previous section. At the same time, due to the high contrast of the color, nanocarbon tracers can help surgeons to correctly distinguish the lymph nodes and normal tissues, reduce the damage to normal tissues and the time of surgical dissection, and increase the number of lymph node dissections. In recent years, nanocarbon tracers have gradually matured for malignant melanoma, breast cancer, thyroid cancer, and some digestive malignant tumors[21]. Nanocarbon lymph node tracers can help surgeons to determine lymph node metastasis to a certain extent, and they can improve the effective removal of lymph nodes during surgery[8,24].
In this study, the injection dose of nanocarbon suspension was 2.0 mL at a total of four sites, with an average of 0.5 mL at each site. According to existing literature, the injection dose of nanocarbon was 0.4-0.6 mL in breast cancer patients[25] and 1.0 mL in thyroid cancer patients[26]. In patients with colorectal cancer, the injection dose of carbon nanoparticles was 1.0 Ml[27]. Considering the deep infiltration of advanced gastric cancer and the thickness of gastric wall tissue compared with thyroid, breast and colorectal tissue, an injection dose < 2.0 mL may lead to unclear lymph node display in some patients. If the dose is > 2.0 mL, some patients may have excessively deep staining due to excessive dosing, which will affect the operation, and the sandwich injection method can be selected for preoperative labeling of carbon nanoparticles[28].
In the present study, the average number of dissected lymph nodes in the experimental group (LTG 34.95 ± 4.81/case; LSG 32.65 ± 3.82/case) was significantly higher than that in the control group (LTG 31.90 ± 4.47/case, LSG 30.88 ± 2.69 /case). Under LTG operation, compared with the control group, the number of lymph nodes dissected at the D1 and D2 stations in the experimental group was significantly better than that in the control group. However, under LSG operation, the number of lymph nodes dissected at the D1 station showed no significant difference between the two groups, and the number of lymph nodes dissected at the D2 station was better than that in the control group. In a study by Cheng et al[29], the number of lymph nodes detected in the nanocarbon group and the non-nanocarbon group was 32.28 ± 4.10/case and 21.28 ± 2.74/case, respectively. In the study of Jia[30], 15484 Lymph nodes were detected in the nanocarbon group and 7963 in the non-nanocarbon group. The average number of lymph nodes detected in each patient in the nanocarbon group and non-nanocarbon group was 31.99 ± 8.99 and 19.81 ± 4.74, respectively. The results in our experimental group are consistent with the previous studies. In our study, there were 180 patients in the experimental group, and no complications such as marker point bleeding or perforation occurred after endoscopic carbon nanolabeling. In addition, there was no significant difference in the operating time, postoperative hospital stay, and incidence of postoperative complications such as postoperative anastomotic fistula, anastomotic bleeding, and obstruction, which proved that the effectiveness and safety of nanocarbon tracers were similar to those in previous studies.
It is worth noting that our study showed that the intraoperative blood loss of the experimental group was less than that of the control group (under the same operation), which may have been due to clearly visualized lymph nodes after preoperative nanocarbon labeling, thus avoiding unnecessary tissue and vascular damage and reducing intraoperative blood loss.
Although preoperative injection of nanocarbon tracer is helpful for lymph node dissection, there is still no consensus or guidelines on the optimal time point for preoperative tracer labeling. By comparison among experimental groups in this study, we found that under the premise of the same operation and the same lymph node station :the results (number of lymph nodes detected and number stained black) of the nanocarbon labeling group 2 and 1 d before surgery were significantly better than those of the labeling group on the day before surgery. At the same time, there was no significant difference in the number of lymph nodes detected between the 2-d and 1-d preoperative labeling groups. This may be because the optimal time for imaging in tissues after injection of carbon nanoparticles is 2-12 h after injection[31], and there was no significant difference compared with previous results.
Our study had some limitations. We only studied the influence of three different labeling time points on the results of lymph node dissection after radical resection of gastric cancer. Further determination of a more accurate labeling time of the tracer needs to be confirmed by more clinical trials. For example, whether better results can be achieved by changing the marker time to 3 or even 4 d before surgery or whether a better result can be achieved between 1 d before and on the day of surgery remains to be answered by more in-depth studies with larger sample sizes.
In conclusion, carbon nanoparticle labeling has a good guiding effect for laparoscopic lymph node dissection of gastric cancer, and is safe and effective. Compared with the control group, preoperative submucosal injection of carbon nanoparticles could significantly improve the detection rate of lymph nodes, which is conducive to pathological staging and subsequent comprehensive antitumor therapy.
Gastric cancer deaths in China account for more than 40% of the global total of gastric cancer deaths in the same period. Reducing cancer-related mortality and improving quality of life is one of the current research focuses, and the number of lymph nodes dissected was an independent factor affecting postoperative staging of gastric cancer. There are still no specific guidelines for when preoperative labeling should be performed, based on many previous works, this work extended the time of preoperative submucosal tracer injection, and discuss whether it is effective.
In order to help surgeons to more lymph node dissection, and improve postoperative pathological staging; At the same time, whether the preoperative labeling time has a certain influence on the number of lymph node dissection was studied.
To study the influence of preoperative carbon nanoparticle labeling combined with radical gastrectomy on the number of dissected lymph nodes and postoperative anti-tumor treatment effect, and to study the influence of preoperative labeling time on the number of dissected lymph nodes.
Retrospective analysis study was performed, all patients were randomly divided into experimental group (preoperative injection of carbon-nano group) and control group (preoperative injection of carbon-nano group) according to the principle of randomization; In the experimental group, according to the different groups of preoperative labeling time, the differences between the groups were studied.
The average number of dissected lymph nodes in the experimental group [34.95 ± 4.81/case in the laparoscopic total gastrectomy (LTG) group; 32.65 ± 3.82/case in the laparoscopic subtotal gastrectomy (LSG) group] was higher than that in the control group (31.90 ± 4.47/case in the LTG group; 30.88 ± 2.69/case in the LSG group, P < 0.05). In comparisons within the experimental group, the experimental results (number of lymph node dissections, number of black-staining lymph nodes) of the nano-carbon labeling group 2 and 1 d before surgery were better than those of the labeling group on the day before surgery (P < 0.05).
(1) Nano-carbon labeling has a good guiding effect on lymph node dissection during laparoscopic gastric cancer, and it is safe and effective; and (2) Compared with the control group, submucosal injection of a carbon tracer in the experimental group at a certain time before surgery can significantly improve the lymph node detection rate (P < 0.05), which is conducive to pathological staging and follow-up anti-tumor comprehensive treatment.
Gastric cancer is the fifth most common cancer in the world, redical operation is still the preferred treatment method for advanced gastric cancer, postoperative lymph node detection rate is one of the major factors affecting PN staging of lymph node metastasis after radical gastrectomy, In order to help the surgeon correctly distinguish the normal tissue from the lymph nodes and dissect lymph nodes as much as possible, endoscopic injection of carbon nanoparticles was selected.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Socea B S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 356] [Article Influence: 89.0] [Reference Citation Analysis (1)] |
| 2. | Lykke J, Roikjaer O, Jess P; Danish Colorectal Cancer Group. The relation between lymph node status and survival in Stage I-III colon cancer: results from a prospective nationwide cohort study. Colorectal Dis. 2013;15:559-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Ohdaira H, Nimura H, Takahashi N, Mitsumori N, Kashiwagi H, Narimiya N, Yanaga K. The possibility of performing a limited resection and a lymphadenectomy for proximal gastric carcinoma based on sentinel node navigation. Surg Today. 2009;39:1026-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Song W, Yuan Y, Wang L, He W, Zhang X, Chen C, Zhang C, Cai S, He Y. The prognostic value of lymph nodes dissection number on survival of patients with lymph node-negative gastric cancer. Gastroenterol Res Pract. 2014;2014:603194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Fang C, Wang W, Deng JY, Sun Z, Seeruttun SR, Wang ZN, Xu HM, Liang H, Zhou ZW. Proposal and validation of a modified staging system to improve the prognosis predictive performance of the 8th AJCC/UICC pTNM staging system for gastric adenocarcinoma: a multicenter study with external validation. Cancer Commun (Lond). 2018;38:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Spinoglio G, Bertani E, Borin S, Piccioli A, Petz W. Green indocyanine fluorescence in robotic abdominal surgery. Updates Surg. 2018;70:375-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Hagen ME, Diaper J, Douissard J, Jung MK, Buehler L, Aldenkortt F, Barcelos GK, Morel P. Early Experience with Intraoperative Leak Test Using a Blend of Methylene Blue and Indocyanine Green During Robotic Gastric Bypass Surgery. Obes Surg. 2019;29:949-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Li Z. Lymph node mapping in rabbit liver cancer with nanocarbon and methylene blue injecta. Asian Pac J Trop Med. 2013;6:400-403. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Degiuli M, De Manzoni G, Di Leo A, D'Ugo D, Galasso E, Marrelli D, Petrioli R, Polom K, Roviello F, Santullo F, Morino M. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol. 2016;22:2875-2893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 98] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 10. | Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol. 2005;12:981-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114-7124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 12. | Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14:317-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Ichikura T, Ogawa T, Chochi K, Kawabata T, Sugasawa H, Mochizuki H. Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J Surg. 2003;27:330-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH, Noh SH. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687-4693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | In H, Ravetch E, Langdon-Embry M, Palis B, Ajani JA, Hofstetter WL, Kelsen DP, Sano T. The newly proposed clinical and post-neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer. 2018;21:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1575] [Cited by in F6Publishing: 1814] [Article Influence: 259.1] [Reference Citation Analysis (0)] |
| 17. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 18. | Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Cancer. 2002;94:2862-2866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Eom BW, Joo J, Park B, Kim YW. Reply to questions in response to "improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer". Surgery. 2014;156:737-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kitagawa Y, Fujii H, Mukai M, Kubota T, Ando N, Watanabe M, Ohgami M, Otani Y, Ozawa S, Hasegawa H, Furukawa T, Kumai K, Ikeda T, Nakahara T, Kubo A, Kitajima M. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Ameri. 2000;80:; 1799-1809. [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 220] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Kitagawa Y, Fujii H, Mukai M, Kubo A, Kitajima M. Sentinel lymph node mapping in esophageal and gastric cancer. Cancer Treat Res. 2005;127:123-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Deng J, Liu J, Wang W, Sun Z, Wang Z, Zhou Z, Xu H, Liang H. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Chin J Cancer Res. 2018;30:477-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Jiang Z. Method and experience of lymph node examination after gastrectomy with D2 Lymphadenectomy for gastric cancer. Zhongguo Weichang Waike Zazhi. 2019;22:1671-0274. [Cited in This Article: ] |
| 24. | Marubini E, Bozzetti F, Miceli R, Bonfanti G, Gennari L; Gastrointestinal Tumor Study Group. Lymphadenectomy in gastric cancer: prognostic role and therapeutic implications. Eur J Surg Oncol. 2002;28:406-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ma JJ, Zhang DB, Zhang WF, Wang X. Application of Nanocarbon in Breast Approach Endoscopic Thyroidectomy Thyroid Cancer Surgery. J Laparoendosc Adv Surg Tech A. 2020;30:547-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Liu F, Zhu Y, Qian Y, Zhang J, Zhang Y. Recognition of sentinel lymph nodes in patients with papillary thyroid cancer by nano-carbon and methylene blue. Pak J Med. 33: 1485-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Yao ZT, Bai QW, Cao XG. Discussion on the role of preoperative endoscopic nano carbon labeling in gastrointestinal cancer surgery. Zhongguo Neikuijing Zazhi. 26:37-40. [Cited in This Article: ] |
| 28. | Wang R, Mo S, Liu Q, Zhang W, Zhang Z, He Y, Cai G, Li X. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: a prospective randomized controlled trial. Jpn J Clin Oncol. 2020;50:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Cheng K, Zhuang J, Li BD, Liu YG, Wang JB, Li D, Feng WY, Xu DL, Zhang HL. Application of Subserosal Injection of Carbon Nanoparticle Lymphatic Tracer in Laparoscopic Assisted Radical Gastrectomy for Advanced Gastric Cancer. Chinese Journal of General Basic and Clinical. 23:1460-3. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Jia RB. Study on nano-carbon tracer on prognosis of patients with gastric cancer. 2018. Available from: http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=D01537436. [Cited in This Article: ] |
| 31. | Shicai W. Application advances of carbon nanomaterial in cancer diagnosis and therapy. Zhongguo Jieruyingxiang Zhiliaoxue. 2018;15:112-115. [Cited in This Article: ] |