Copyright
©The Author(s) 2022.
World J Clin Cases. Oct 6, 2022; 10(28): 9970-9984
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.9970
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.9970
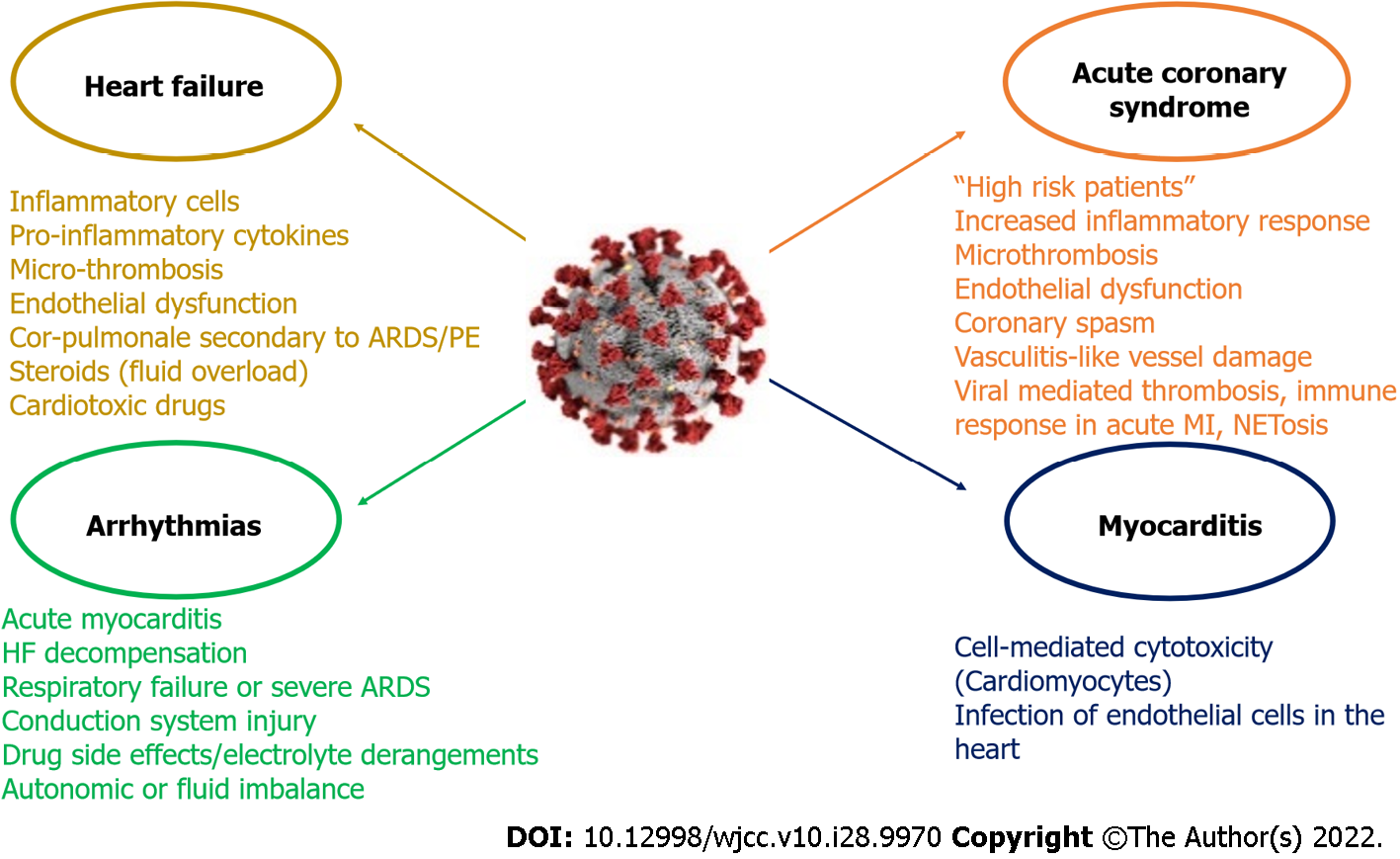
Figure 4 Coronavirus disease 2019 related cardiovascular complications and its main mechanisms.
It is proposed that two different groups of coronavirus disease 2019 (COVID-19) patients with acute coronary syndrome (ACS) exist. The first group includes “high risk” patients (i.e. pre-existing atherosclerosis, a history of heart failure (HF) or multiple cardiovascular risk factors), who are prone to develop acute myocardial injury. In the second group patients may lack previous cardiovascular history or classic risk factors for coronary disease and the excessive inflammatory response to the primary infection is the main mechanism. Nonetheless the two groups may share common pathophysiological mechanisms (microthrombosis, endothelial dysfunction, coronary spasm, vasculitis-like vessel damage). The main mechanisms associated with thrombosis and immune response presented in COVID-19 patients with ACS include viral mediated thrombosis, immune response in acute myocardial infarction (MI) and NETosis. Acute myocarditis has been reported as a possible complication in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients. The entrance of SARS-CoV-2 in cardiac myocyte finally leads to the production of primed CD8+ T lymphocytes, which migrate to the cardiomyocytes and may cause myocardial inflammation through cell-mediated cytotoxicity. SARS-CoV-2 can also cause myocardial damage via the infection of endothelial cells in the heart. COVID-19 patients may develop HF due to infiltration of heart by inflammatory cells, destructive action of pro-inflammatory cytokines, micro-thrombosis and new onset or aggravated endothelial dysfunction and respiratory failure. Other possible mechanisms include cor-pulmonale secondary to acute respiratory distress syndrome (ARDS) or pulmonary embolism (PE), the use of steroids which can cause fluid overload, and the administration of cardiotoxic drugs (i.e. hydroxychloroquine). SARS-CoV-2 can engender arrhythmias through direct myocardial damage causing acute myocarditis or through HF decompensation or secondary, through respiratory failure or severe ARDS. Direct viral myocardial injury to the conduction system can also predispose to arrhythmogenesis. Other possible mechanisms include drug side effects, electrolyte derangements, and autonomic or fluid imbalance. NET: Neutrophil extracellular traps.
- Citation: Xanthopoulos A, Bourazana A, Giamouzis G, Skoularigki E, Dimos A, Zagouras A, Papamichalis M, Leventis I, Magouliotis DE, Triposkiadis F, Skoularigis J. COVID-19 and the heart. World J Clin Cases 2022; 10(28): 9970-9984
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/9970.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.9970