Copyright
©The Author(s) 2022.
World J Clin Cases. Jul 26, 2022; 10(21): 7483-7494
Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7483
Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7483
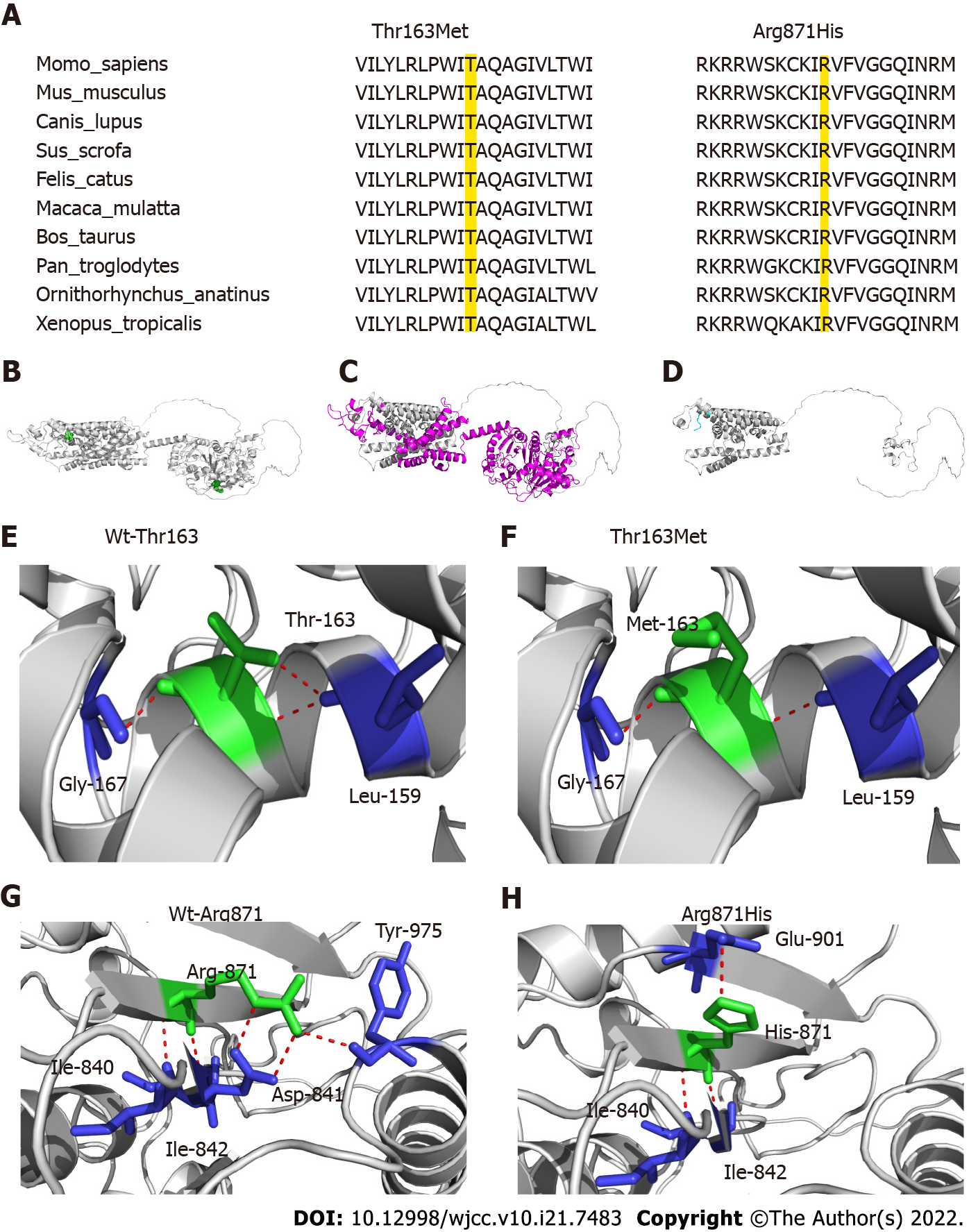
Figure 3 Schematic presentation of the structure of SLC12A3.
A: Thr163 and Arg871 are highly conserved amino acids in various species (the locations colored yellow); B: Overview of the locations of Thr163 and Arg871 in the global three-dimensional structure of the protein. Thr163 and Arg871 are shown in green spheres, and the global protein structure is shown in the cartoon model; C and D: The mutation Ile393fs causes missing of some protein regions and domains (magenta), and transfer of MPPLAPAW* novel sequence (cyan); E and F: Analysis of changes in hydrogen bonds for the Thr163Met mutation. The key amino acids are shown as sticks and H-bonds are shown as red dotted line. One H-bond is destroyed when Thr163 is replaced by Met; G and H: The mutation Arg871His will cause large changes in the H-bond network, destruction of H-bond interaction with Asp841 and Tyr975, and generation of a new interaction with Glu901.
- Citation: Qin YZ, Liu YM, Wang Y, You C, Li LN, Zhou XY, Lv WM, Hong SH, Xiao LX. Novel compound heterozygous mutation of SLC12A3 in Gitelman syndrome co-existent with hyperthyroidism: A case report and literature review. World J Clin Cases 2022; 10(21): 7483-7494
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7483.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7483