Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6784
Peer-review started: March 23, 2022
First decision: May 12, 2022
Revised: May 16, 2022
Accepted: June 17, 2022
Article in press: June 17, 2022
Published online: July 16, 2022
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. In some patients, COVID-19 is complicated with myocarditis. Early detection of myocardial injury and timely intervention can significantly improve the clinical outcomes of COVID-19 patients. Although endomyocardial biopsy (EMB) is currently recognized as the ‘gold standard’ for the diagnosis of myocarditis, there are large sampling errors, many complications and a lack of unified diagnostic criteria. In addition, the clinical methods of treating acute and chronic COVID-19-related myocarditis are different. Cardiac magnetic resonance (CMR) can evaluate the morphology of the heart, left and right ventricular functions, myocardial perfusion, capillary leakage and myocardial interstitial fibrosis to provide a noninvasive and radiation-free diagnostic basis for the clinical detection, efficacy and risk assessment, and follow-up observation of COVID-19-related myocarditis. However, for the diagnosis of COVID-19-related myocarditis, the Lake Louise Consensus Criteria may not be fully applicable. COVID-19-related myocarditis is different from myocarditis related to other viral infections in terms of signal intensity and lesion location as assessed by CMR, which is used to visualize myocardial damage, locate lesions and quantify pathological changes based on various sequences. Therefore, the standardized application of CMR to timely and accurately evaluate heart injury in COVID-19-related myocarditis and develop rational treatment strategies could be quite effective in improving the prognosis of patients and preventing potential late-onset effects in convalescent patients with COVID-19.
Core tip: This review aims to explore the frontiers of Coronavirus disease 2019 (COVID-19)-related myocarditis as assessed by Cardiac magnetic resonance (CMR) and compare the similarities and differences in CMR signs between COVID-19-related myocarditis and myocarditis related to other viral infections. COVID-19-related myocarditis is different from myocarditis related to other viral infections in SI and lesion location as assessed by CMR. The Lake Louise Consensus Criteria are not fully applicable to COVID-19-related myocarditis. CMR is expected to visualize myocardial damage, locate lesions and quantify pathological changes based on various sequences with the benefit of staged diagnosis and treatment in COVID-19-related myocarditis.
- Citation: Luo Y, Liu BT, Yuan WF, Zhao CX. Frontiers of COVID-19-related myocarditis as assessed by cardiovascular magnetic resonance. World J Clin Cases 2022; 10(20): 6784-6793
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6784.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6784
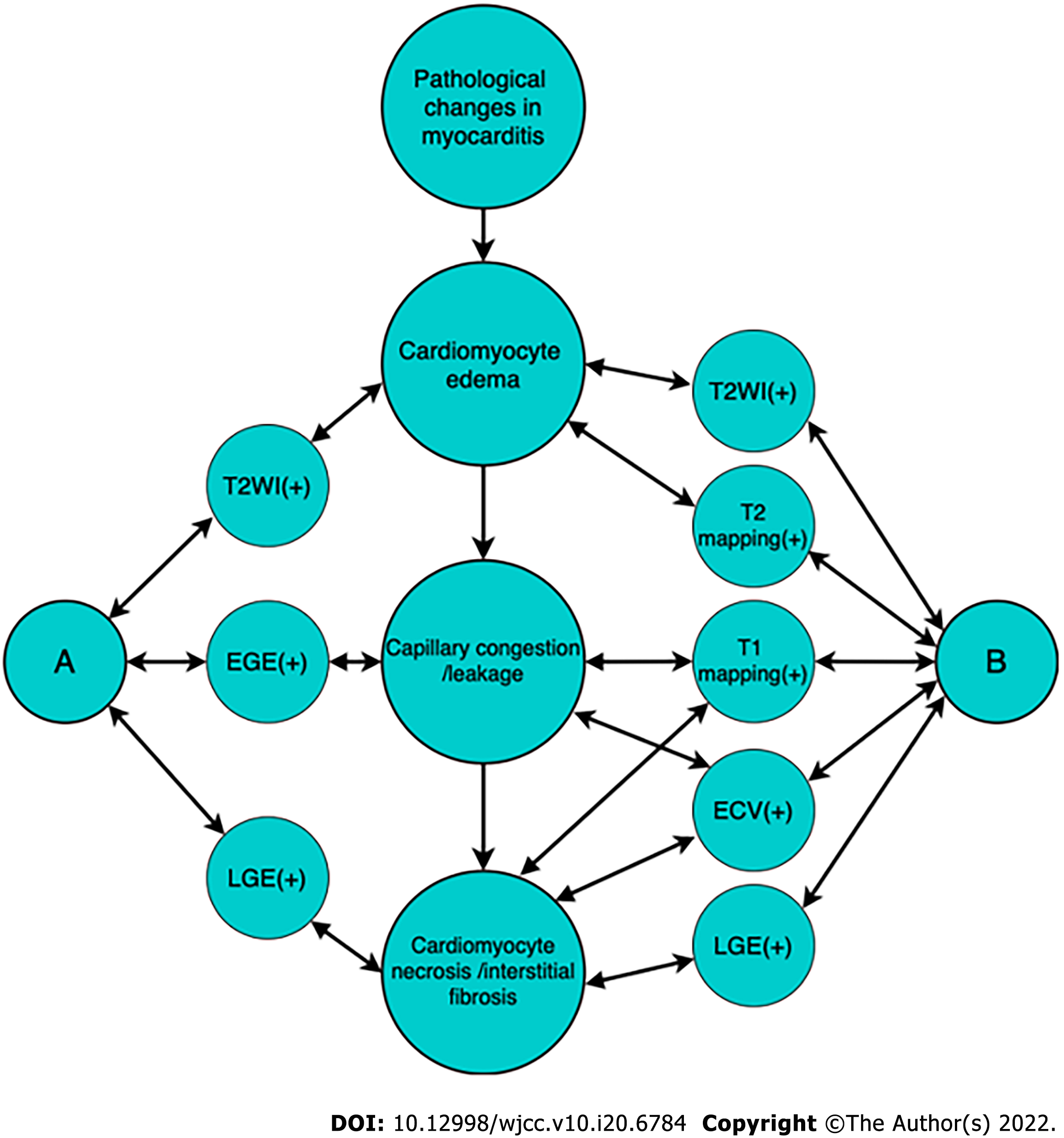
In patients with coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the main clinical manifestations of patients are fever and cough. In patients with severe cases of COVID-19, acute respiratory distress syndrome and respiratory failure can occur[1,2]. In some patients, COVID-19 is often complicated with myocarditis[3]. In general, the severity of COVID-19 is proportional to the degree of cardiac injury. Early detection of myocardial injury and timely intervention can significantly improve the clinical outcomes of COVID-19 patients[4,5]. Although endomyocardial biopsy (EMB) is currently recognized as the ‘gold standard’ for the diagnosis of myocarditis, there are large sampling errors, many complications and a lack of unified diagnostic criteria[6,7]. In addition, the clinical methods of treating acute and chronic COVID-19-related myocarditis are different[8,9]. Cardiac magnetic resonance (CMR) can evaluate the morphology of the heart, left and right ventricular functions, myocardial perfusion, capillary leakage and myocardial interstitial fibrosis to provide a noninvasive and radiation-free diagnostic basis for the clinical detection, efficacy and risk assessment, and follow-up observation in COVID-19-related myocarditis in one step[10-14]. The Lake Louise Consensus Criteria (2009) have been widely used in the CMR diagnosis of myocarditis[15]. The new guidelines (2018) updated and supplemented the imaging techniques and parameters to improve the accuracy of CMR in the diagnosis of myocarditis[16]. The pathological changes in myocarditis as assessed by CMR are depicted in Figure 1. However, for the diagnosis of COVID-19-related myocarditis, the Lake Louise Consensus Criteria may not be fully applicable. This review aims to explore the frontiers of COVID-19-related myocarditis as assessed by CMR and compare the similarities and differences in CMR signs between COVID-19-related myocarditis and myocarditis related to other viral infections. We used ['COVID-19' or 'SARS-CoV-2'] and [‘myocarditis’ or ‘myocardial inflammation’] and ['MR' or ‘MRI’ or ‘magnetic resonance’] as the search terms and mainly searched the relevant academic articles included in PubMed.
Compared with other viral infection-related diseases, COVID-19 is associated with a high risk of death from cardiovascular complications, with an unclear pathophysiological mechanism[17,18]. Current studies[18-20] propose that the potential pathogenesis of cardiovascular injury mainly includes: (1) Direct virus damage; (2) Hypoxia and an imbalance of myocardial oxygen supply and demand; (3) Cytokine storms; and (4) Abnormal coagulation. COVID-19-related cardiac injury may involve many pathways[21], among which immune response disorder, microcirculation disorder and the side effects of antiviral drugs may be the main causes of COVID-19 myocardial injury. SARS-CoV-2 can lead to a systemic inflammatory response and immune and coagulation dysfunction after host infection. Angiotensin-converting enzyme-2 is widely distributed in the heart; however, SARS-CoV-2 can still enter host cardiomyocytes. Although studies have shown that virus particles can be observed in cardiomyocytes, there is no evidence of virus particle or SARS-CoV-2 gene expression in cardio
For acute and chronic COVID-19-related myocarditis, the clinical applications of specific remedies and symptomatic treatments are different[25]. Determining how to accurately stage COVID-19-related myocarditis is essential. According to the pathological development and outcomes, COVID-19-related myocarditis involves an acute stage, a subacute stage, a recovery stage, a chronic stage and a sequelae stage[26-29]. However, in the choice of treatment plans for patients with viral myocarditis, COVID-19-related myocarditis is often reducible to just an acute stage and a chronic stage for symptomatic treatment. To alleviate cardiac insufficiency and improve the survival rate, intravenous gamma globulin can be used in the treatment of acute viral myocarditis, especially in children[30]. Immunosuppressants cannot be prescribed for patients with myocarditis not confirmed by pathology, and routine use of immunosuppressants in patients with acute myocarditis is not advocated. However, for patients with chronic myocarditis complicated with complete atrioventricular block or cardiogenic shock, immunosuppressants can be used in sufficient amounts[31]. In addition, for patients with chronic myocarditis complicated with heart failure, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, β receptor blockers and diuretics are recommended to be used reasonably to reduce inflammatory reactions and prevent ventricular remodelling[32]. Therefore, CMR not only can reflect the histopathological characteristics of myocardial injury but also is the most promising technical method to determine the clinical stage of COVID-19-related myocarditis.
The main pathological changes in COVID-19-related acute myocarditis include capillary congestion and leakage, cardiomyocyte oedema and necrosis. Myocardial oedema and decreased systolic function in early myocarditis are reversible injuries[16]. Thus, timely intervention contributes to significantly reducing mortality in acute myocarditis. T2 weighted imaging (T2WI) is sensitive to oedema, which shows high signal intensity (SI). Short time of inversion recovery in T2WI (T2-STIR) is often used to improve the contrast between the oedematous area and the normal myocardium. Oedema is a specific marker of reversible injury in acute myocarditis and usually occurs 2-3 weeks before severe cardiomyocyte damage[33]. Therefore, the presence of oedema is helpful to distinguish between acute and chronic COVID-19-related myocarditis. T2WI is recommended as a characteristic index to describe focal and diffuse oedema in COVID-19-related myocarditis[34]. During the treatment of acute myocarditis, the decrease in T2WI SI can be applied as a marker of oedema remission to achieve the purpose of monitoring the condition. However, other diseases, such as sarcomatosis and immune rejection of a heart transplant, also show similar T2WI SI, and the evaluation is subjective; therefore, the high T2WI SI may be not specific. T2 mapping generates different T2WI scans based on different T2 relaxation times by steady-state free precession (SSFP) and then calculates the corresponding pixels of each image by fitting the parameter equation. Therefore, the SI of each image can reflect different echo times to realize the quantification and analysis of T2 values. It is worth mentioning that the SSFP sequence can reduce various unstable factors, such as the long T2 signals generated by slow blood flow during scanning and motion artefacts caused by poor breath holding. T2 mapping is a more accurate, rapid and quantitative method for detecting myocardial oedema to compensate for the defects of traditional T2-STIR. The detection rate of myocardial oedema by T2 mapping is much higher than that by T2-STIR. T2 mapping is quite accurate for defining the scope of myocardium infection and reflecting myocardial oedema and is positively correlated with high-sensitivity troponin in the acute stage of COVID-19-related myocarditis[35]. In addition, scanning methods of gadolinium contrast enhancement in CMR include early gadolinium enhancement (EGE) and late gadolinium enhancement (LGE). Because gadolinium contrast agent is an extracellular contrast agent that cannot pass through the complete biofilm, the two techniques are applied to detect the different characteristics of myocardial injury of COVID-19-related myocarditis: EGE is mostly used to reflect tissue congestion, which is the characteristic of an active inflammatory reaction; LGE indicates irreversible heart injury, such as myocardial necrosis and interstitial fibrosis[36]. The acute course of COVID-19-related myocarditis is approximately 4 weeks, mainly including myocardial cell membrane rupture and myocardial tissue dissolution. With the increase in capillary blood flow and vascular leakage, gadolinium contrast agent quickly distributes to the intercellular space. Generally, at 3-5 min after injection, EGE is shown as high SI with a rapid increase in gadolinium contrast agent concentration in myocardial tissue. In the early stage of acute COVID-19-related myocarditis, a single inflammatory lesion gradually develops and spreads into multiple lesions, and the accurate application of EGE contributes to a sensitive diagnosis within the first 2 weeks after infection. A return to normal of EGE within 1 month after acute myocardial inflammatory injury indicates that left ventricular function has recovered well. Myocardial perfusion imaging after severe COVID-19 also demonstrates regional ischaemia rather than global blood flow reduction[37]. Nevertheless, the biggest limitation of EGE is that it is unable to quantify SI accurately. Arrhythmia, motion artefacts caused by poor breath holding, and fast heart rate in infants may lead to failure to evaluate EGE SI. Thus, quantitative techniques such as T1 mapping and extracellular volume (ECV) analysis are recommended to evaluate COVID-19-related myocarditis. Image acquisition of T1 mapping occurs at different inversion times of the same phase of multiple cardiac cycles to directly and quantitatively measure the T1 value of each voxel of the myocardium and display the difference in the T1 value of the myocardium. Compared with T1-weighted imaging, the application of T1 mapping is expected to reduce the subjectivity of traditional qualitative evaluation and increase the repeatability of CMR evaluation[38]. Similar to the T2 mapping value, the native T1 mapping value also increases in acute myocarditis. It has been reported that children with COVID-19-related myocarditis show mild subepicardial LGE, suggesting diffuse interstitial oedema and myocardial injury; after immunomodulatory treatment, the oedema is relieved, and the corresponding native T1 value decreases[39,40].
LGE is expected to evaluate cardiomyocyte necrosis and fibrosis in irreversible myocardial damage caused by myocarditis after SARS-CoV-2 infection[41-43]. The content of free water is relatively low in cardiomyocytes, so oedema is not obvious, and the detection rate of T2WI is not high in chronic COVID-19-related myocarditis. Because gadolinium contrast agent can significantly reduce the T1 relaxation time and increase the SI in the myocardial injured area, LGE is more sensitive for detecting oedema than T2WI[44]. Due to their broken cell membranes, necrotic cardiomyocytes absorb gadolinium contrast agent and show a higher SI than normal cardiomyocytes. Gadolinium contrast agent accumulates in necrotic cardiomyocytes (early stage of necrosis) and myocardium affected by interstitial fibrosis (late stage of necrosis) within a few minutes after intravenous injection, and its content exceeds that of normal myocardial tissue. Thus, LGE indicates typical "delayed enhancement" in the early and late stages of myocardial necrosis, respectively, with characteristic significance in the detection of cardiomyocyte necrosis. The LGE-positive location in COVID-19-related myocarditis is not only similar to that in myocarditis related to other viral infections, often involving the inferior wall and lateral wall of the left ventricle, but also appears in the ventricular septum and free wall at the base and middle of the left ventricle[45-50]. Importantly, compared with other viral infections, the area of LGE caused by SARS-CoV-2 infection of the myocardium is more extensive, but the SI of T2WI and LGE may not be obviously high[51]. Moreover, Eiros et al[52] and Inciardi et al[53] evaluated COVID-19-related myocarditis through CMR, and they found that the patients had not only myocardial interstitial oedema and diffuse LGE but also ventricular dysfunction, pericarditis and pericardial effusion; CMR in a male patient with COVID-19 mild pericarditis showed focal oedema in the lateral, anterior, inferior and apical wall of the left ventricle with epicardial involvement[54]; and a female patient with acute COVID-19-related pericarditis and cardiac tamponade presented in CMR with subepicardial LGE of the anterolateral wall of the left ventricle[55]. Interestingly, adolescents and children infected with SARS-CoV-2 may present the clinical characteristics of Kawasaki disease and multisystem inflammatory syndrome (MIS)[56,57]. Most children with MIS showed no obvious LGE but myocardial oedema strain and an abnormal myocardium, suggesting that focal myocardial necrosis or fibrosis was rare, but there was a lack of research for long-term follow-up and re-examination[40,58]. Therefore, whether the prognosis of children and adolescents infected with SARS-CoV-2 is favourable needs further multicentre and big-data research. However, due to the correlation between the detection rate of LGE and the severity of myocardial damage, the sensitivity of COVID-19-related myocarditis detection is limited by the area of myocarditis. The smaller the area of myocarditis, the less likely it is to be detected by LGE. In addition, LGE also shows high SI in certain cardiomyopathies and myocardial amyloidosis, so its diagnostic specificity for COVID-19-related myocarditis is not high. COVID-19-related myocarditis needs to be comprehensively analysed by combining T2WI, EGE, T1 mapping, ECV analysis, diffusion tensor imaging (DTI) and other quantitative parameters. At present, the clinical diagnosis of myocardial fibrosis often depends on EMB, and T1 mapping has been gradually popularized and applied to reduce the potential risks associated with EMB. T1 mapping is expected to evaluate the prognosis of COVID-19-related myocarditis, and ECV analysis is an important technical method in coordination with T1 mapping[59]. Myocardial fibrosis is the main feature of COVID-19-related myocarditis in the chronic stage and one of the pathological mechanisms leading to ventricular remodelling. Fortunately, the expansion of the myocardial extracellular matrix is reversible, and early clinical medication is expected to prevent the proliferation of fibrosis. Therefore, T1 mapping and ECV analysis not only help to determine the clinical stage of COVID-19-related myocarditis but also provide the necessary diagnostic basis for guiding treatment through quantitative analysis of myocardial extracellular matrix volume[60]. It is worth mentioning that DTI is a technology based on DWI to quantify the anisotropy of water molecules and measure the degree and direction of water molecule diffusion[61]. DTI is expected to analyse the data of water molecular diffusion in three-dimensional space to noninvasively observe the damage severity of myocardial tissue infected by SARS-CoV-2 and evaluate whether the myocardial structure integrity has been lost[62]. Clinicians should be vigilant against the possible late-onset effects of convalescent patients with COVID-19 and use the CMR multiparameter model to dynamically and quantitatively evaluate myocardial fibrosis in follow-up.
In approximately 31% of patients, COVID-19-related myocarditis can be complicated with secondary myocardial ischaemia[63]. SARS-CoV-2 first damages the endothelial cells of blood vessels in different human tissues and organs. Diffuse endothelial inflammation causes endothelial dysfunction and microvascular dysfunction and then leads to vasoconstriction, blood hypercoagulability and thrombosis, resulting in secondary myocardial ischaemia and myocardial infarction[64]. Different from computed tomography angiography and echocardiography to evaluate coronary artery dilatation and rupture in COVID-19, CMR is mainly used to evaluate the severity of myocardial injury and detect the thrombus at the apex[45,65]. Interestingly, LGE of certain patients infected with SARS-CoV-2 showed that the infarct area was 3/4 the area of the anterior wall, and T2WI-STIR showed cardiomyocyte oedema in the corresponding location, suggesting acute myocardial infarction, but the patients had no symptoms[26]. Secondary myocardial infarction caused by SARS-CoV-2 infection in children is often accompanied by cardiomyocyte oedema and coronary artery dilatation, which is difficult to distinguish from acute Kawasaki disease. This indicates a poor prognosis if LGE becomes positive in the follow-up. Furthermore, unlike myocarditis related to other viral infections, COVID-19-related myocarditis is often secondary to right ventricular enlargement and dysfunction, which may be related to pulmonary hypertension and acute pulmonary embolism caused by SARS-CoV-2 infection[66]. Right ventricular dysfunction was independently associated with all-cause mortality in patients with COVID-19-related myocarditis[67]. Ventricular remodelling may gradually induce heart failure and related complications, resulting in a poor prognosis and significantly increased mortality. Huang et al[48] found that a long-term decline in right ventricular function parameters, such as the right ventricular ejection fraction, lasted in patients with COVID-19-related myocarditis. Some studies have also found that left ventricular dilation and decreased left ventricular ejection fraction occurred in convalescent patients with COVID-19[23,68]. Although SARS-CoV-2 appears to cause less damage to the left ventricle, there may be sustained damage, so its long-term results need to be followed up.
T1 mapping is not recommended to be independently applied in the diagnosis of COVID-19-related myocarditis because several factors probably cause changes in the T1 value. In addition, DTI is prone to errors in tracking crossed myocardium fibres in chronic myocarditis. Moreover, artificial intelligence techniques have relatively matured in assisting in the diagnosis and prognosis analysis of COVID-19, including COVID-19-related pneumonia, but not in COVID-19-related myocarditis. In this mini-review, the majority of the available data were from case reports and observational studies that focused on CMRI performed in the setting of acute COVID-19-related myocarditis. We need more data from cohort studies to support the findings in the setting of chronic COVID-19-related myocarditis. Findings from multicentre and large-scale research projects on COVID-19, such as CISCO-19, will contribute to guiding the clinical application of CMR in COVID-19-related myocarditis and heart injury[12,69].
COVID-19-related myocarditis is different from myocarditis related to other viral infections in SI and lesion location as assessed by CMR. Although the Lake Louise Consensus Criteria are not fully applicable to COVID-19-related myocarditis, they are still the most authoritative diagnostic guidelines and need to be further supplemented. CMR is expected to visualize myocardial damage, locate lesions and quantify pathological changes based on various sequences with the benefit of staged diagnosis and treatment. Therefore, the standardized application of CMR to timely and accurately evaluate heart injury in COVID-19-related myocarditis and develop rational treatment strategies could be quite effective in improving the prognosis of patients and preventing potential late-onset effects in convalescent patients with COVID-19.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed SK, Iraq; Fatima M; Tazegul G, Turkey S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12459] [Article Influence: 3114.8] [Reference Citation Analysis (1)] |
| 2. | Chen S, Ren LZ, Ouyang HS, Liu S, Zhang LY. Necessary problems in re-emergence of COVID-19. World J Clin Cases. 2021;9:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Doyen D, Moceri P, Ducreux D, Dellamonica J, Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 4. | Cau R, Bassareo PP, Mannelli L, Suri JS, Saba L. Imaging in COVID-19-related myocardial injury. Int J Cardiovasc Imaging. 2021;37:1349-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Jaiswal V, Almas T, Peng Ang S, David S, Shama N, Storozhenko T, Lnu K, Parmar G, Qaiser S, Naz S, Jaiswal A, Malik J, Symptomatology, prognosis and clinical findings of STEMI as a ramification of COVID-19: A systematic review and proportion meta-analysis. Ann Med Surg (Lond). 2022;76:103429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Caforio ALP, Baritussio A, Basso C, Marcolongo R. Clinically Suspected and Biopsy-Proven Myocarditis Temporally Associated with SARS-CoV-2 Infection. Annu Rev Med. 2022;73:149-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Tanacli R, Doeblin P, Götze C, Zieschang V, Faragli A, Stehning C, Korosoglou G, Erley J, Weiss J, Berger A, Pröpper F, Steinbeis F, Kühne T, Seidel F, Geisel D, Cannon Walter-Rittel T, Stawowy P, Witzenrath M, Klingel K, Van Linthout S, Pieske B, Tschöpe C, Kelle S. COVID-19 vs. Classical Myocarditis Associated Myocardial Injury Evaluated by Cardiac Magnetic Resonance and Endomyocardial Biopsy. Front Cardiovasc Med. 2021;8:737257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Sagar S, Liu PP, Cooper LT Jr, Myocarditis. Lancet. 2012;379(9817):738-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 9. | Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 215] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 10. | Sanghvi SK, Schwarzman LS, Nazir NT. Cardiac MRI and Myocardial Injury in COVID-19: Diagnosis, Risk Stratification and Prognosis. Diagnostics (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, Li H, Xu L. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Gorecka M, McCann GP, Berry C, Ferreira VM, Moon JC, Miller CA, Chiribiri A, Prasad S, Dweck MR, Bucciarelli-Ducci C, Dawson D, Fontana M, Macfarlane PW, McConnachie A, Neubauer S, Greenwood JP, investigators C-H, Demographic, multi-morbidity and genetic impact on myocardial involvement and its recovery from COVID-19: protocol design of COVID-HEART-a UK, multicentre, observational study. J Cardiovasc Magn Reson. 2021;23(1):77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, Leith D, Shetye A, Ariff B, Bell R, Captur G, Coleman M, Goldring J, Gopalan D, Heightman M, Hillman T, Howard L, Jacobs M, Jeetley PS, Kanagaratnam P, Kon OM, Lamb LE, Manisty CH, Mathurdas P, Mayet J, Negus R, Patel N, Pierce I, Russell G, Wolff A, Xue H, Kellman P, Moon JC, Treibel TA, Cole GD, Fontana M. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866-1878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 238] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 14. | Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, Elliott MD, Ferrari VA, Friedrich MG, Lawton C, Manning WJ, Ordovas K, Plein S, Powell AJ, Raman SV, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P; International Consensus Group on Cardiovascular Magnetic Resonance in M; Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1600] [Cited by in F6Publishing: 1627] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 16. | Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158-3176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 762] [Cited by in F6Publishing: 1114] [Article Influence: 222.8] [Reference Citation Analysis (0)] |
| 17. | Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, Aimo A, Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27:251-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 18. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart Rhythm. 2020;106:1132-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 19. | Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT, Jr Chahal CAA. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 465] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 20. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 1181] [Article Influence: 295.3] [Reference Citation Analysis (1)] |
| 21. | Wu L, O'Kane AM, Peng H, Bi Y, Motriuk-Smith D, Ren J. SARS-CoV-2 and cardiovascular complications: From molecular mechanisms to pharmaceutical management. Biochem Pharmacol. 2020;178:114114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A, Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J, 2020; 41(19): 1861-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 396] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 23. | Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1240] [Cited by in F6Publishing: 1351] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 24. | Panchal A, Kyvernitakis A, Mikolich JR, Biederman RWW. Contemporary use of cardiac imaging for COVID-19 patients: a three center experience defining a potential role for cardiac MRI. Int J Cardiovasc Imaging. 2021;37:1721-1733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76 (10):1244-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 274] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 26. | Tschöpe C, Sherif M, Anker MS, Geisel D, Kuehne T, Kelle S. COVID-19-convalescence phase unmasks a silent myocardial infarction due to coronary plaque rupture. ESC Heart Fail. 2021;8:971-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Clark DE, Dendy JM, Li DL, Crum K, Dixon D, George-Durrett K, Parikh AP, Wassenaar JW, Hughes SG, Soslow JH. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC). J Cardiovasc Magn Reson. 2021;23:106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Italia L, Tomasoni D, Bisegna S, Pancaldi E, Stretti L, Adamo M, Metra M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front Cardiovasc Med. 2021;8:713560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 29. | Keri VC, Hooda A, Kodan P, R LB, Jorwal P, Wig N, Intricate interplay between Covid-19 and cardiovascular diseases. Rev Med Virol, 2021; 31(4): e2188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Robinson JL, Hartling L, Crumley E, Vandermeer B, Klassen TP. A systematic review of intravenous gamma globulin for therapy of acute myocarditis. BMC Cardiovasc Disord. 2005;5:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Camargo PR, Okay TS, Yamamoto L, Del Negro GM, Lopes AA. Myocarditis in children and detection of viruses in myocardial tissue: implications for immunosuppressive therapy. Int J Cardiol. 2011;148:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Oussalah A, Gleye S, Clerc Urmes I, Laugel E, Callet J, Barbé F, Orlowski S, Malaplate C, Aimone-Gastin I, Caillierez BM, Merten M, Jeannesson E, Kormann R, Olivier JL, Rodriguez-Guéant RM, Namour F, Bevilacqua S, Losser MR, Levy B, Kimmoun A, Gibot S, Thilly N, Frimat L, Schvoerer E, Guéant JL. Long-term ACE Inhibitor/ARB Use Is Associated With Severe Renal Dysfunction and Acute Kidney Injury in Patients With Severe COVID-19: Results From a Referral Center Cohort in the Northeast of France. Clin Infect Dis. 2020;71:2447-2456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G; Cardiac Magnetic Resonance" Working Group of the Italian Society of C; Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis. J Am Coll Cardiol. 2019;74(20):2439-2448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Breitbart P, Koch A, Schmidt M, Magedanz A, Lindhoff-Last E, Voigtländer T, Schmermund A, Mehta RH, Eggebrecht H. Clinical and cardiac magnetic resonance findings in post-COVID patients referred for suspected myocarditis. Clin Res Cardiol. 2021;110:1832-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Galea N, Marchitelli L, Pambianchi G, Catapano F, Cundari G, Birtolo LI, Maestrini V, Mancone M, Fedele F, Catalano C, Francone M. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a Cardiovascular Magnetic Resonance study. J Cardiovasc Magn Reson. 2021;23:68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging. 2018;11:1583-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 37. | Thornton GD, Shetye A, Knight DS, Knott K, Artico J, Kurdi H, Yousef S, Antonakaki D, Razvi Y, Chacko L, Brown J, Patel R, Vimalesvaran K, Seraphim A, Davies R, Xue H, Kotecha T, Bell R, Manisty C, Cole GD, Moon JC, Kellman P, Fontana M, Treibel TA. Myocardial Perfusion Imaging After Severe COVID-19 Infection Demonstrates Regional Ischemia Rather Than Global Blood Flow Reduction. Front Cardiovasc Med. 2021;8:764599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Li S, Duan X, Feng G, Sirajuddin A, Yin G, Zhuang B, He J, Xu J, Yang W, Wu W, Sun X, Zhao S, Wang H, Teng Z, Lu M. Multiparametric Cardiovascular Magnetic Resonance in Acute Myocarditis: Comparison of 2009 and 2018 Lake Louise Criteria With Endomyocardial Biopsy Confirmation. Front Cardiovasc Med. 2021;8:739892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Oberweis ML, Codreanu A, Boehm W, Olivier D, Pierron C, Tsobo C, Kohnen M, Abdelrahman TT, Nguyen NT, Wagner K, de la Fuente Garcia I. Pediatric Life-Threatening Coronavirus Disease 2019 With Myocarditis. Pediatr Infect Dis J. 2020;39:e147-e149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H. Cardiac MRI in Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Radiology. 2020;297:E283-E288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 41. | Bustin A, Sridi S, Gravinay P, Legghe B, Gosse P, Ouattara A, Rozé H, Coste P, Gerbaud E, Desclaux A, Boyer A, Prevel R, Gruson D, Bonnet F, Issa N, Montaudon M, Laurent F, Stuber M, Camou F, Cochet H. High-resolution Free-breathing late gadolinium enhancement Cardiovascular magnetic resonance to diagnose myocardial injuries following COVID-19 infection. Eur J Radiol. 2021;144:109960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Myhre PL, Heck SL, Skranes JB, Prebensen C, Jonassen CM, Berge T, Mecinaj A, Melles W, Einvik G, Ingul CB, Tveit A, Berdal JE, Røsjø H, Lyngbakken MN, Omland T. Cardiac pathology 6 months after hospitalization for COVID-19 and association with the acute disease severity. Am Heart J. 2021;242:61-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Kim JY, Han K, Suh YJ. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID-19: a systematic review and meta-analysis. J Cardiovasc Magn Reson. 2021;23:100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Raafs AG, Ghossein MA, Brandt Y, Henkens MTHM, Kooi ME, Vernooy K, Spaanderman MEA, Gerretsen S, van Santen S, Driessen RGH, Knackstedt C, van der Horst ICC, van Bussel BCT, Heymans SRB, Ghossein-Doha C; MaastrICCht collaborators. Cardiovascular outcome 6 months after severe coronavirus disease 2019 infection. J Hypertens. 2022;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Gravinay P, Issa N, Girard D, Camou F, Cochet H. CMR and serology to diagnose COVID-19 infection with primary cardiac involvement. Eur Heart J Cardiovasc Imaging. 2021;22:133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Abdelazeem B, Borcheni M, Alnaimat S, Mallikethi-Reddy S, Sulaiman A. Persistent Cardiac Magnetic Resonance Imaging Features of Myocarditis Detected Months After COVID-19 Infection. Cureus. 2021;13:e14250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Esposito A, Palmisano A, Natale L, Ligabue G, Peretto G, Lovato L, Vignale D, Fiocchi F, Marano R, Russo V. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC Cardiovasc Imaging. 2020;13:2462-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging. 2020;13:2330-2339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 362] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 49. | Pavon AG, Meier D, Samim D, Rotzinger DC, Fournier S, Marquis P, Monney P, Muller O, Schwitter J. First Documentation of Persistent SARS-Cov-2 Infection Presenting With Late Acute Severe Myocarditis. Can J Cardiol. 2020;36:1326.e5-1326.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Beşler MS, Arslan H. Acute myocarditis associated with COVID-19 infection. Am J Emerg Med. 2020;38:2489.e1-2489.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 51. | Luetkens JA, Isaak A, Öztürk C, Mesropyan N, Monin M, Schlabe S, Reinert M, Faron A, Heine A, Velten M, Dabir D, Boesecke C, Strassburg CP, Attenberger U, Zimmer S, Duerr GD, Nattermann J. Cardiac MRI in Suspected Acute COVID-19 Myocarditis. Radiol Cardiothorac Imaging. 2021;3:e200628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Eiros R, Barreiro-Pérez M, Martín-García A, Almeida J, Villacorta E, Pérez-Pons A, Merchán S, Torres-Valle A, Sánchez-Pablo C, González-Calle D, Pérez-Escurza O, Toranzo I, Díaz-Peláez E, Fuentes-Herrero B, Macías-Álvarez L, Oliva-Ariza G, Lecrevisse Q, Fluxa R, Bravo-Grande JL, Orfao A, Sánchez PL; CCC (cardiac COVID-19 healthcare workers) investigators. Pericardial and myocardial involvement after SARS-CoV-2 infection: a cross-sectional descriptive study in healthcare workers. Rev Esp Cardiol (Engl Ed). 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M, Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1111] [Cited by in F6Publishing: 1233] [Article Influence: 308.3] [Reference Citation Analysis (0)] |
| 54. |
Monmeneu JV, Dominguez Mafe E, Andres Soler J, Ventura Perez B, Solsona Caravaca J, Broseta Torres R, Garcia-Gonzalez P, Higueras Ortega L, Lopez-Lereu MP, Maceira AM, Subacute perimyocarditis in a young patient with COVID-19 infection.
|
| 55. | Dalen H, Holte E, Guldal AU, Hegvik JA, Stensaeth KH, Braaten AT, Mjølstad OC, Rossvoll O, Wiseth R. Acute perimyocarditis with cardiac tamponade in COVID-19 infection without respiratory disease. BMJ Case Rep. 2020;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Mavrogeni SI, Kolovou G, Tsirimpis V, Kafetzis D, Tsolas G, Fotis L. The importance of heart and brain imaging in children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C). Rheumatol Int. 2021;41:1037-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Li DL, Davogustto G, Soslow JH, Wassenaar JW, Parikh AP, Chew JD, Dendy JM, George-Durrett KM, Parra DA, Clark DE, Hughes SG. Characteristics of COVID-19 Myocarditis With and Without Multisystem Inflammatory Syndrome. Am J Cardiol. 2022;168:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, Cleary A, Stewart K, Adhvaryu K, Savis A, Kabir SR, Uy MP, Heard H, Peacock K, Miller O, Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22(8):896-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 59. | Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7:667-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 150] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 60. | Greulich S, Klingel K. COVID-19 and Myocarditis: Findings from Cardiac Magnetic Resonance Imaging and Endomyocardial Biopsies. Hamostaseologie. 2021;41:366-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Chowdhary A, Garg P, Das A, Nazir MS, Plein S, Cardiovascular magnetic resonance imaging: emerging techniques and applications. Heart. 2021;107(9):697-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Paddock S, Tsampasian V, Assadi H, Mota BC, Swift AJ, Chowdhary A, Swoboda P, Levelt E, Sammut E, Dastidar A, Broncano Cabrero J, Del Val JR, Malcolm P, Sun J, Ryding A, Sawh C, Greenwood R, Hewson D, Vassiliou V, Garg P. Clinical Translation of Three-Dimensional Scar, Diffusion Tensor Imaging, Four-Dimensional Flow, and Quantitative Perfusion in Cardiac MRI: A Comprehensive Review. Front Cardiovasc Med. 2021;8:682027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, Wolff A, Moon JC, Xue H, Kellman P, Patel N, Fontana M. COVID-19: Myocardial Injury in Survivors. Circulation. 2020;142:1120-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 64. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4227] [Cited by in F6Publishing: 4297] [Article Influence: 1074.3] [Reference Citation Analysis (0)] |
| 65. | Bernardi N, Calvi E, Cimino G, Pascariello G, Nardi M, Cani D, Faggiano P, Vizzardi E, Nunzia DM, Marco M. COVID-19 Pneumonia, Takotsubo Syndrome, and Left Ventricle Thrombi. JACC Case Rep. 2020;2:1359-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V, Ingallina G, Napolano A, Finazzi R, Ruggeri A, Ajello S, Melisurgo G, Camici PG, Scarpellini P, Tresoldi M, Landoni G, Ciceri F, Scandroglio AM, Agricola E, Cappelletti AM. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 67. | Moody WE, Mahmoud-Elsayed HM, Senior J, Gul U, Khan-Kheil AM, Horne S, Banerjee A, Bradlow WM, Huggett R, Hothi SS, Shahid M, Steeds RP. Impact of Right Ventricular Dysfunction on Mortality in Patients Hospitalized With COVID-19, According to Race. CJC Open. 2021;3:91-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Erdol MA, Ozbay MB, Yayla C, Arslan H, Isiksalan Ozbulbul N, Ozcan Cetin EH, Karanfil M, Erdoğan M, Demirtas K, Ertem AG, Akcay AB. Cardiac involvement in MRI in young population after COVID-19: A single tertiary center experience. Echocardiography. 2021;38:1327-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 69. | Mangion K, Morrow A, Bagot C, Bayes H, Blyth KG, Church C, Corcoran D, Delles C, Gillespie L, Grieve D, Ho A, Kean S, Lang NN, Lennie V, Lowe DJ, Kellman P, Macfarlane PW, McConnachie A, Roditi G, Sykes R, Touyz RM, Sattar N, Wereski R, Wright S, Berry C. The Chief Scientist Office Cardiovascular and Pulmonary Imaging in SARS Coronavirus disease-19 (CISCO-19) study. Cardiovasc Res. 2020;116:2185-2196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |