Copyright
©The Author(s) 2022.
World J Clin Cases. Jul 6, 2022; 10(19): 6360-6369
Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6360
Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6360
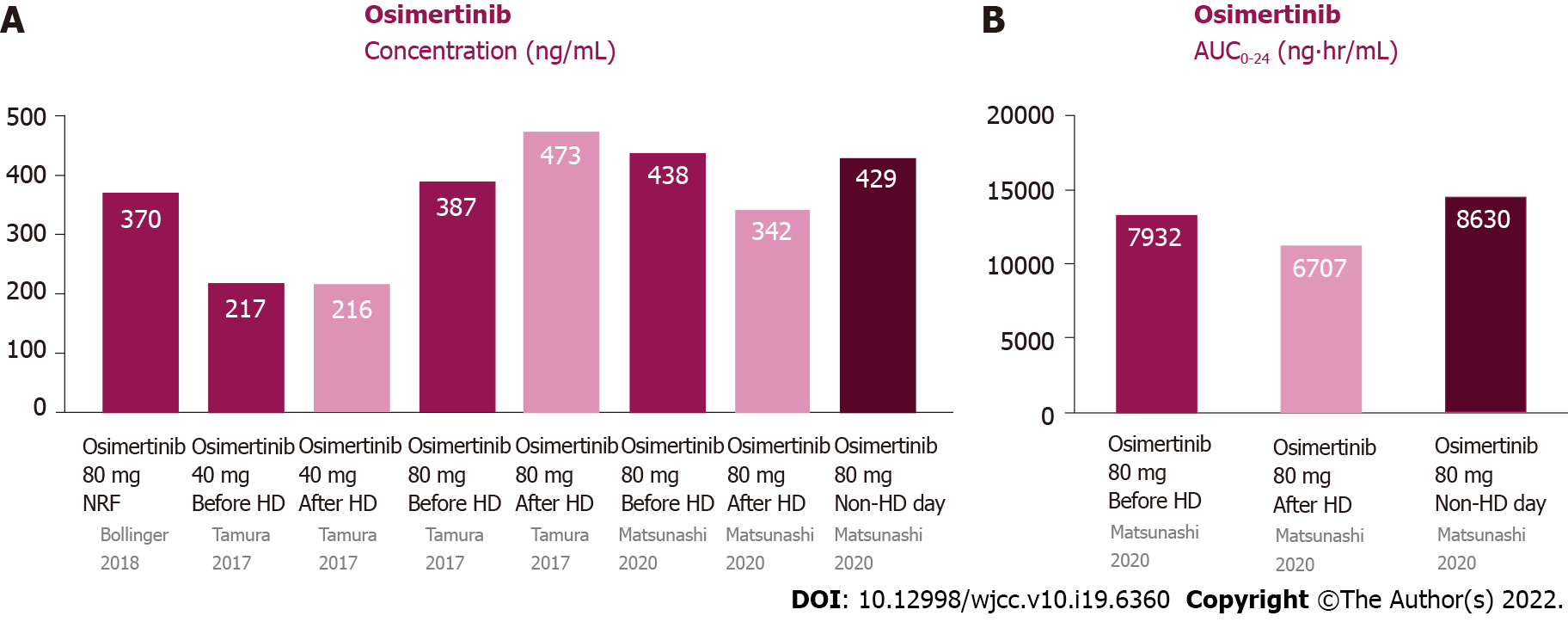
Figure 5 Pharmacokinetics of osimertinib in patients undergoing hemodialysis and those with normal renal function.
A: Plasma concentrations of osimertinib were similar among pre-HD, post-HD, and non-HD days; B: The AUC0–24 of osimertinib were similar among pre-HD, post-HD, and non-HD days. AUC0-24: Area under the curve of the plasma concentration from 0 to 24 h; HD: Hemodialysis; NRF: Normal renal function.
- Citation: Lan CC, Hsieh PC, Huang CY, Yang MC, Su WL, Wu CW, Wu YK. Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis. World J Clin Cases 2022; 10(19): 6360-6369
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6360