Copyright
©The Author(s) 2022.
World J Clin Cases. Jul 6, 2022; 10(19): 6360-6369
Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6360
Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6360
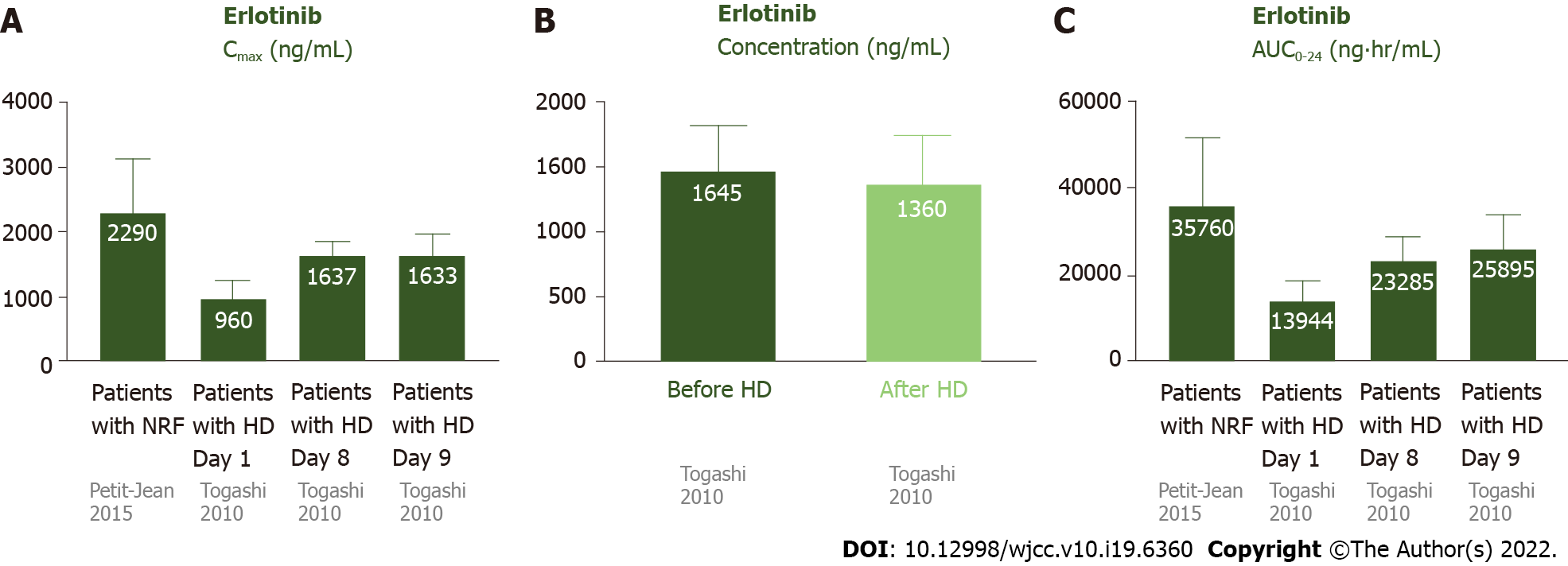
Figure 3 Pharmacokinetics of erlotinib in patients undergoing hemodialysis and those with normal renal function.
A: The Cmax of erlotinib was 2290 ± 840 ng/mL in patients with NRF. For the patients undergoing HD, the Cmax was 1638 ± 206 ng/mL on day 8 and 1638 ± 206 ng/mL on day 9 (after steady state). The Cmax was similar in patients with NRF and undergoing HD; B: The plasma concentrations of erlotinib were similar before and after HD; C: The AUC0-24 was 35760 ± 15720 ng· hr/mL in patients with NRF. For the patients undergoing HD, the AUC0-24 was 23285 ± 5338 ng· hr/mL on day 8 and 25895 ± 7747 ng· hr/mL on day 9. The AUC0-24 was similar in patients with NRF and undergoing HD. AUC0-24: Area under the curve of the plasma concentration from 0 to 24 h; Cmax: Maxium concentration; Ctrough: Trough concentration; HD: Hemodialysis; NRF: Normal renal function.
- Citation: Lan CC, Hsieh PC, Huang CY, Yang MC, Su WL, Wu CW, Wu YK. Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis. World J Clin Cases 2022; 10(19): 6360-6369
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6360