Copyright
©The Author(s) 2022.
World J Clin Cases. Jun 26, 2022; 10(18): 6009-6020
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6009
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6009
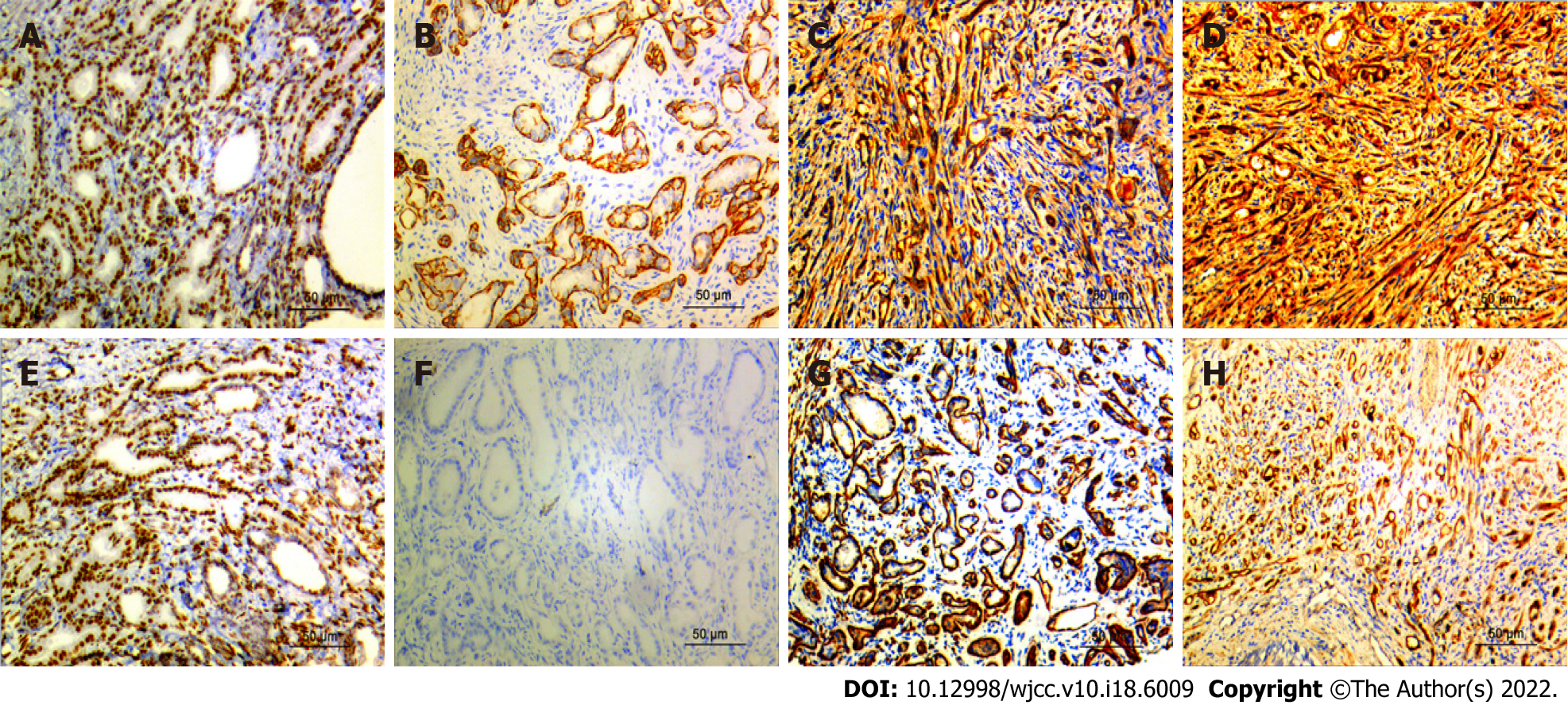
Figure 4 Immunohistochemistry results.
A: AR was strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); B: Calponin is positively expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); C: CK5/6 is positively expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); D: CKH was strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); E: P63 was moderately expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); F: P504S is not expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); G: SMA is moderately expressed in prostatic sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); H: S100 is strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm).
- Citation: Feng RL, Tao YP, Tan ZY, Fu S, Wang HF. Prostate sclerosing adenopathy: A clinicopathological and immunohistochemical study of twelve patients. World J Clin Cases 2022; 10(18): 6009-6020
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6009